Figure 1.
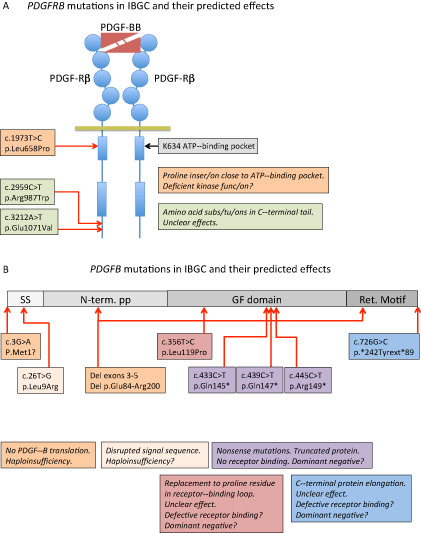
Schematic representation of the mutations reported to date in PDGFB and PDGFRB and their predicted effects on protein function. A. A schematic representation of PDGF‐Rβ with PDGF‐BB bound to the extracellular ligand‐binding domain. Circles in PDGF‐Rβ indicate immunoglobulin domains and boxes represent kinase domains. The three idiopathic basal ganglia calcification (IBGC) mutations reported to date are indicated by type and approximate position. Note that one of the mutations is predicted to be damaging to the receptor functions, as it introduces a proline residue close to the ATP‐binding pocket of the receptor kinase. Proline residues are known to have strong effects on the three‐dimensional (3D) structure in this region. B. The PDGF‐B precursor protein is indicated in different shades of gray. SS, signal sequence for secretion; N‐term. PP, amino‐terminal pro‐peptide; GF domain, growth factor domain; Ret. Motif, heparan sulfate proteoglycan‐binding extracellular matrix retention motif. The nature and approximate position of the mutations are indicated and their effects described in boxes with the same color. Haploinsufficiency indicates that mere loss of one functional copy of the PDGFB gene is disease causing. For the mutants with putative residual cysteine residues engaged in ligand dimerization, a dominant‐negative effect might be conceived, which in theory predicts a 75% reduction of the amount produced functional PDGF‐BB.
