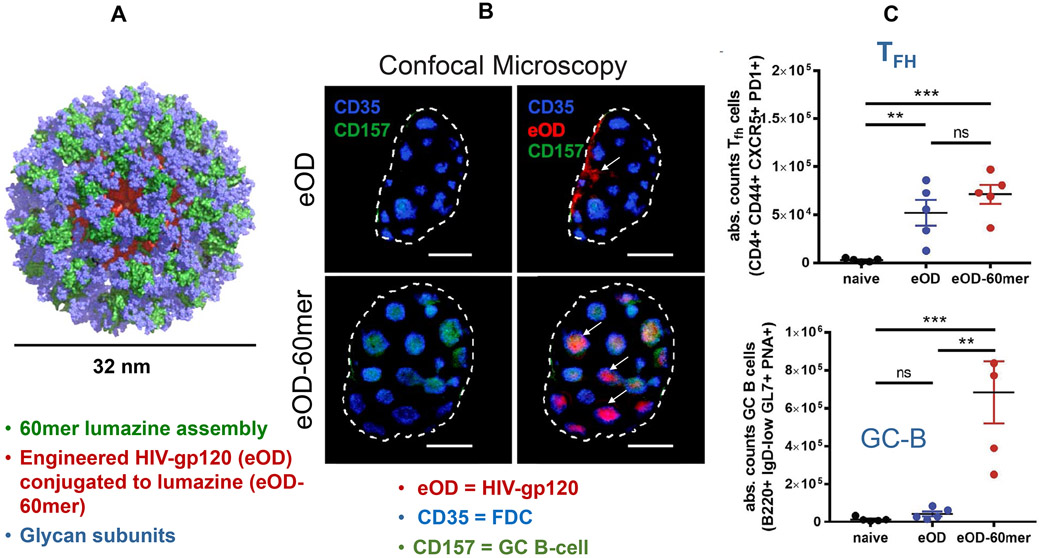
Figure 11. Use of nanoparticle glycosylation to target lymph node germinal centers.
Particulate HIV immunogens are more capable of activating low-affinity germline precursor B-cells than monomeric antigens, in addition to promoting TFH cooperation with germinal center B-cells.146 This capability can be used for nano-enabled enhancement of neutralizing antibody responses. A. The engineered outer domain (eOD) of the soluble HIV-gp120 monomer was formulated into ~32-nm nanoparticles by fusion to a glycan-rich bacterial protein, lumazine synthase, which self-assembles into a 60-mer (eOD-60mer).146 B. Confocal microscopy of a lymph node showing that soluble monomers enter the subcapsular sinus of the lymph node but do not gain access to germinal centers (darker blue). During challenge with monomers, the centers exhibit sparse follicular dendritic cells (FDC) (lighter blue) and B-cells (green). However, administration of the eOD-60mer demonstrates access to the germinal center, loaded with FDC and B-cells.146 The mechanism of germinal center recruitment has been ascribed to glycans on the particle surface binding to mannose-binding protein. This signal triggers complement activation, which supports polymerized antigen access to the germinal center.146 C. Quantitative expression of the number of TFH and B cells in the germinal centers. Reprinted with permission from ref 146. Copyright 2019 AAAS.