Abstract
Background:
Paclitaxel-containing devices (PTXD) significantly reduce reintervention in patients with symptomatic femoropopliteal peripheral artery disease (PAD). A recent aggregate-data meta-analysis reported increased late mortality in PAD patients treated with PTXD. We performed an individual patient data (IPD) meta-analysis to evaluate mortality.
Methods:
Manufacturers of FDA approved and commercially available devices in the United States provided de-identified IPD for independent analysis. Cox proportional hazards one-stage meta-analysis models using intention-to-treat (ITT) methods were used for the primary analysis. A secondary analysis of additionally recovered missing vital status data was performed. The impact of control crossover to PTXD, cause-specific mortality and drug dose-mortality were assessed.
Results:
2,185 subjects and 386 deaths from eight PTXD trials with 4-year median follow-up were identified. The primary analysis indicated a 38% (95% confidence interval [CI], 6% to 80%) increased relative mortality risk, corresponding to 4.6% absolute increase, at 5 years associated with PTXD use. Control and treatment arm loss to follow-up and withdrawal were 24% and 23%, respectively. With inclusion of recovered vital status data the excess relative mortality risk was 27% (95% CI, 3% to 58%). This observation was consistent across various scenarios, including as-treated analyses, with no evidence of increased risk over time with PTXD. Mortality risk tended to be increased for all major causes of death. There were no subgroup differences. No drug dose-mortality association was identified.
Conclusions:
This IPD meta-analysis, based on the most complete available data set of mortality events from PTXD randomized controlled trials, identified an absolute 4.6% increased mortality risk associated with PTXD use.
Keywords: Peripheral arterial disease, drug coated balloons, drug eluting stents, mortality, meta-analysis
Introduction
Paclitaxel-coated angioplasty balloons and paclitaxel-eluting stents have demonstrated effectiveness in treating femoropopliteal peripheral artery disease (PAD) by reducing reintervention through 5-year follow-up.1, 2 However, an aggregate-level meta-analysis3 raised concerns of increased all-cause late mortality, manifest ≥ 2 years after exposure to paclitaxel-containing devices (PTXD). In response to this report, the US Food and Drug Administration (FDA) issued three advisory letters to healthcare providers 4–6 suggesting use of alternate non-paclitaxel based endovascular devices and heightened surveillance of patients exposed to these devices until additional data were available. To overcome the limitations of meta-analyses using aggregate-level published data, VIVA Physicians collaborated with the four US manufacturers of PTXDs to transfer individual patient data from their regulatory Investigational Device Exemption (IDE) randomized controlled trials to an independent third-party medical research organization for an IPD meta-analysis.
Methods
Protocol, Study Selection, Data Collection
Because of the sensitive nature of the data collected for these analyses, requests to access the datasets from qualified researchers trained in human subject confidentiality protocols may be sent to the individual industry members research organizations noted below. The final content of a Statistical Analysis Plan (SAP) (Supplement Materials) was approved by a four-member independent multispecialty Data Steering Committee assembled by VIVA Physicians and was based on formal discussions with the U.S. Food and Drug Administration (FDA) with review and approval by industry members. Following agreed upon FDA guidance, eligible studies were required to be randomized controlled trials (RCTs) with individual patient data available from study sponsors and ≥2 years of follow-up evaluating US-licensed PTXDs (IN.PACT™ Admiral™, Medtronic, Santa Rosa, CA.; Lutonix® Paclitaxel-Coated Balloon, Bard/Becton Dickinson, Covington, GA; Stellarex™ Paclitaxel-Coated Balloon, Philips Medical, The Netherlands; and Zilver® PTX® Stent, Cook Medical, Bloomington, IN) for use in the femoropopliteal artery compared with a control arm using a non-drug-coated balloon or a bare metal stent. The patient population included subjects with PAD involving the superficial femoral and/or popliteal artery with intermittent claudication or ischemic rest pain (Rutherford Class 2 to 4). Only devices commercially available in the United States as of March 2019 were included in this analysis to inform US regulatory decision-making. NAMSA (Minneapolis, MN) independently performed all statistical analyses.
Sponsors provided anonymized data on mortality and baseline characteristics for each patient in the RCT(s) and were responsible for data monitoring, reviewing and quality. Individual patient data were reviewed for integrity and re-coded as necessary to facilitate harmonization of variables and definitions across datasets. Previously unreported vital status data received after the approval of the SAP were included in sensitivity analyses. Institutional Review Board (IRB) approval was not required for this individual patient-level meta-analysis.
Outcome and Analysis Methods
The primary outcome was all-cause mortality. The measure of effect was the hazard ratio (HR), with values greater than 1 indicating an increased risk of mortality associated with PTXD treatment relative to bare-metal stent and balloon treatment. Confidence intervals that exclude a hazard ratio of 1 are considered statistically significant.
All analyses were pre-specified in the SAP. The primary analysis utilized an intent-to-treat approach (ITT) with subjects analyzed according to their original randomized assignment.
The primary outcome was summarized using a one-stage meta-analysis approach.7 Methods of survival analysis were used to account for the number and timing of deaths, as well as the specific amount of observed follow-up for subjects surviving. The primary model was a Cox proportional hazards model stratified by study, with a normally distributed random study effect. Kaplan-Meier analyses employed Hall-Wellner 95% confidence bands.8 We assessed the effects of control subject crossover to PTXD, informative censoring, “as-treated” populations (analyses according to the actual treatment received at the index procedure), cause-specific mortality, and additional longer-term follow-up data for vital status based on efforts to collect participant outcomes beyond the original study data collection. An “as-treated” analysis adjusted for covariates to account for possible bias due to departure from the ITT principle was performed. The adjustment was made via propensity score stratification based on quintiles of the propensity score estimated separately for each study. Covariates for the propensity score model were pre-specified in the SAP (Table S1). Subgroup analyses based on pre-randomization factors were performed to further explore differences in mortality between study arms by assessing treatment by covariate interactions. Risk of bias was assessed for the set of studies using the Cochrane Collaboration’s tool for assessing risk of bias9.
A potential drug dose-mortality relation was explored. Index procedure nominal load data (μg/mm2) were provided by each sponsor and standardized within each study. Total paclitaxel dose received at the index procedure was post-hoc standardized (log transformed, mean centered and scaled) within each study so that the standardized distribution within each study had a mean of zero and standard deviation of 1. The association of mortality with standardized dose was calculated from a proportional hazards model with stratification by study and propensity score weighting, with a penalized spline to show the shape of the association of dose with mortality risk.10 Analyses included both assessments of exposure categories (controls vs. dose categories based on within-study tertile of exposure, i.e., low/medium/high) and continuous dose. Propensity score adjustment was used to account for potential confounding due to the non-randomized nature of the dose-mortality analysis. The analytic exposure modeling strategy was included in the SAP.
Statistical analyses were conducted using SAS version 9.4 or later (SAS Institute Inc., Cary, NC) and R (version R 3.5.3 or later). Key R packages were used: survival package (version 2.44.1.1), coxme package (version 2.2–10), metafor package (version 2.1.0), metagen package (version 1.0), and forest plot package (version 1.9).
Results
Included studies
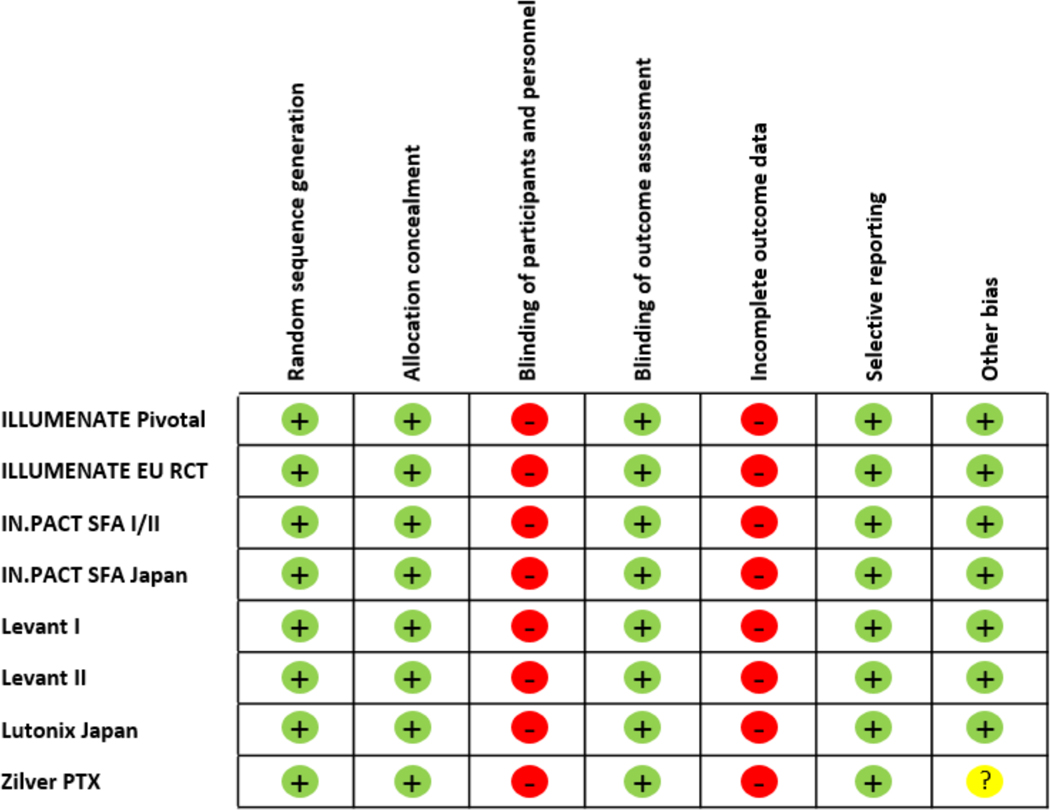
De-identified individual patient data were obtained from 8 RCTs (Figure 1).11 Study characteristics are summarized in Table 1. There were 7 RCTs of paclitaxel-coated balloons and one study of a paclitaxel-eluting stent. A median of 4 years (range 2 to 5 years) of follow-up was obtained. Risk of bias is summarized in Figure 2; there is a high and consistent risk of bias from the open label nature of long-term assessments of vital status as well as the extent of missing data. Of note, 428 subjects (19.6% of the total) exited prematurely from these 8 studies. IPD was not available for two potentially eligible studies: the BATTLE Trial12 (n=181), a RCT comparison of Zilver® PTX® coated stent which included patients with in-stent restenosis and limited six-month follow-up; and the DEBELLUM Trial13 (n=50), a RCT comparison of IN.PACT™ SFA DCB vs. PTA in claudicants with limited one-year follow-up.
Figure 1.
Study Flow Diagram11
Table 1.
Study Characteristics
| Primary Analysis (Original Data) | Updated Data | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | NCT Identifier | Enrollment Time Frame | N Subjects | Allocation Ratio (Treatment: Control) | Median Years Follow-up | Total Patient-Years Follow-up | N Deaths | Unknown Status | Median Years Follow-up | Total Patient-Years Follow-up | N Deaths | Unknown Status |
| ILLUMENATE Pivotal | NCT01858428 | 2013–2015 | 300 | 2:1 | 3.1 | 993.9 | 36 | 34 | 3.1 | 993.9 | 36 | 34 |
| ILLUMENATE EU RCT | NCT01858363 | 2012–2015 | 294 | 3:1 | 4.0 | 1045.7 | 39 | 52 | 4.0 | 1045.7 | 39 | 52 |
| IN.PACT SFA I/II | NCT01175850 NCT01566461 | 2010–2013 | 331 | 2:1 | 4.9 | 1395.1 | 39 | 60 | 4.9 | 1608.1 | 53 | 9 |
| IN.PACT SFA Japan | NCT01947478 | 2013–2015 | 100 | 2:1 | 3.0 | 282.5 | 6 | 8 | 3.0 | 282.5 | 6 | 8 |
| Levant I | NCT00930813 | 2009–2009 | 101 | 1:1 | 2.0 | 181.2 | 9 | 17 | 2.0 | 181.2 | 9 | 17 |
| Levant II | NCT01412541 | 2011–2012 | 476 | 2:1 | 4.9 | 1918.4 | 72 | 78 | 4.9 | 2186.1 | 90 | 20 |
| Lutonix Japan | NCT01816412 | 2013–2014 | 109 | 2:1 | 2.0 | 203.1 | 5 | 5 | 2.0 | 203.1 | 5 | 5 |
| Zilver PTX | NCT00120406 | 2005–2008 | 474 | 1:1 | 4.9 | 1817.7 | 65 | 174 | 6.2 | 3251.9 | 148 | 31 |
| All Studies | 2005–2015 | 2185 | 4.0 | 7837.7 | 271 | 428 | 4.8 | 9752.5 | 386 | 176 | ||
The studies were conducted in the following countries: ILLUMENATE Pivotal: United States, Austria; ILLUMENATE EU RCT : Germany; IN.PACT SFA: United States, Germany; IN.PACT Japan: Japan; Levant I: Belgium, Germany; Levant II: United States, Austria, Belgium, Germany; Lutonix Japan: Japan; Zilver PTX: United States, Germany, Japan
Figure 2. Risk of Bias Assessment.
Risk of bias is assessed for components of each study with green (+) indicating low risk, red (−) indicating high risk, and yellow (?) indicating uncertain risk. The unique design of the Zilver PTX study, with a secondary randomization to paclitaxel, increased the potential for bias with regards to the choice of analysis and the interpretation
Our analyses include data for 2,185 patients of whom 1,382 were assigned to PTXD and 803 to control. There were 271 deaths included in the primary analysis, and 386 in the updated analysis. Baseline clinical characteristics are summarized in Table S2. Average age by study ranged from 67 to 75 years and 24% to 41% of subjects were female. Most subjects had claudication (91%) and 94% were Rutherford category ≤ 3.
Primary Results
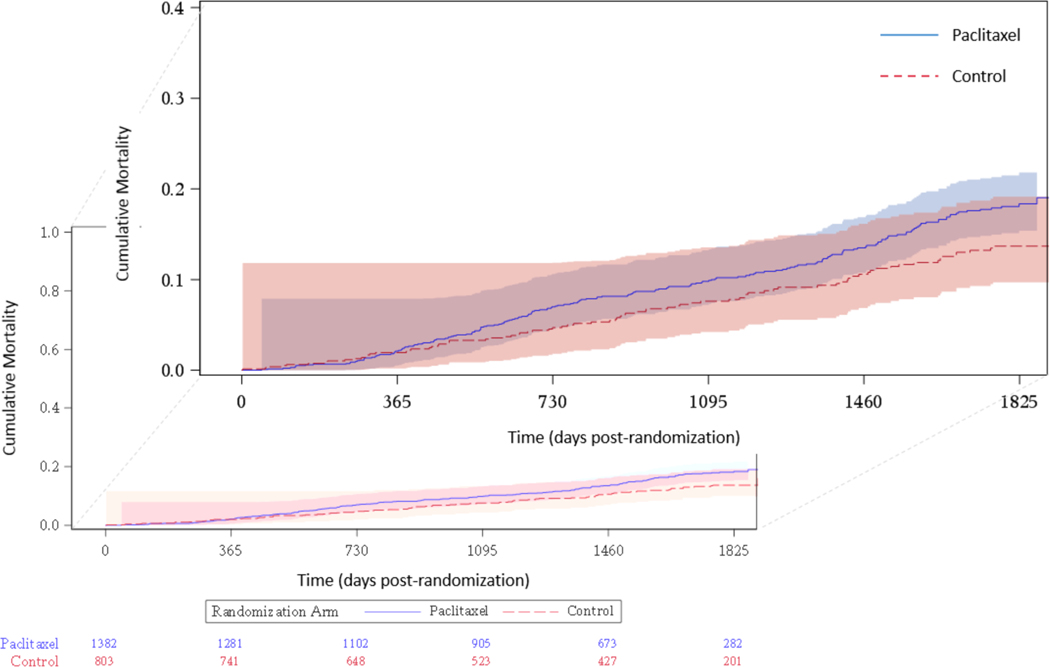
Results from the primary analysis model are displayed in Figure 3. The overall hazard ratio (HR) was 1.38 (95% confidence interval [CI], 1.06 to 1.80) indicating a 38% relative increase in mortality for PTXD relative to control. There was no evidence of a violation of the proportional hazards assumption (p=0.87). Figure 4 and Table S3 summarize the Kaplan-Meier analysis for all studies. Mortality for the PTXD and control groups through 5 years was 18.3% for PTXD and 13.7% for control, an absolute difference of 4.6%.
Figure 3. Primary Analysis Showing Results of ITT Meta-analysis.
Study heterogeneity, I2 = 7%, from the two-stage model
Figure 4. Kaplan-Meier 5-year Cumulative Mortality.
Two deaths occurred after 5 years and thus are not displayed. The two events are incorporated into all of the analyses, including the Kaplan-Meier estimates.
Sensitivity Analyses
Table 2 shows the results of pre-specified sensitivity analyses as well as results from updated analyses. In “as treated” analyses, the unadjusted (HR 1.36, 95% CI, 1.04 to 1.78) and adjusted results (HR 1.37, 95% CI, 1.04 to 1.80) based on a one-stage approach were similar to our primary analysis.
Table 2.
Sensitivity Analyses
| Lost to Follow-up/Withdrawn | |||
|---|---|---|---|
| Models | Effect* (95% CI) | Paclitaxel | Control |
| VIVA original analyses | 23% | 24% | |
| Primary, intent-to-treat | 1.38 (1.06, 1.80) | ||
| As treated, unadjusted | 1.36 (1.04, 1.78) | ||
| As treated, adjusted | 1.37 (1.04, 1.80) | ||
| Censoring at control crossover to paclitaxel | 1.31 (1.00, 1.72) | ||
| Missing data sensitivity / weighted analysis | 1.36 (1.05, 1.77) | ||
| Fixed effect two-stage meta-analysis | 1.36 (1.05, 1.77) | ||
| Random effects two-stage meta-analysis | 1.34 (1.01, 1.78) | ||
| DCB only devices | 1.25 (0.92, 1.69) | ||
| Zilver PTX 2nd randomization instead of primary | 1.19 (0.89, 1.60) | ||
| Additional analyses | |||
| JAHA original, December 2018 (RR) | 1.93 (1.27, 2.93) | NR | NR |
| JAHA update, May 2019 (RR) | 1.62 (1.20, 2.17) | NR | NR |
| FDA, as treated (RR) | 1.72 (1.22, 2.38) | 24% | 24% |
| VIVA, additional follow-up #1 (Late May 2019) | 1.30 (1.03, 1.63) | 15% | 16% |
| VIVA, additional follow-up #2 (August 2019) | 1.27 (1.03, 1.58) | 9% | 10% |
Estimate is hazard ratio (HR) unless indicated otherwise
CI: Confidence interval; DCB: Drug-coated balloon; PTX: Paclitaxel; JAHA: Journal of the American Heart Association; RR: Relative Risk; NR: Not reported
JAHA/FDA analyses are based only on studies with 4–5 years of follow-up; VIVA analyses are based on survival analysis methods incorporating all available follow-up. Data for the FDA analysis and VIVA primary analysis were provided to the FDA in advance of the Circulatory Systems Advisory Panel meeting. The additional follow-up data sets in the VIVA analysis were based on additional follow-up for vital status that was provided by manufacturers.
A “tipping point” analysis revealed a sensitivity of results to the potential pattern of vital status among subjects with missing data (Table S4). In an attempt to resolve the impact of missing data, beyond the original study data used for our primary analysis, sponsor efforts to further determine vital status of exited patients (from IN.PACT™ SFA I/II, Levant II, and Zilver® PTX®) through direct queries to clinical sites and searches of the National Death Index (NDI) further reduced loss to follow-up to 9.8% for control subjects and 9.3% for PTXD subjects. This resulted in attenuation of the mortality hazard ratio (HR 1.27, 95% CI, 1.03 to 1.58).
Analyses censoring control subjects at crossover to PTXD (HR 1.31, 95% CI, 1.00 to 1.72) and an analysis to address potential informative censoring14 (using inverse probability of censoring weighting, HR 1.36, 95% CI, 1.05 to 1.77) were consistent with the primary findings (Table 2 and Figure S1).
Two-stage meta-analysis using both fixed effect and random effects models produced estimates similar to our primary model7 The random effects model had a lower confidence limit of 1.01, in contrast to the lower bound of 1.06 for the primary one-stage meta-analysis model, which also employed random effects. We pre-specified use of a restricted maximum likelihood approach; other methods produced a lower estimate of study variability, and results more similar to the one-stage approach. Study heterogeneity estimated from the two-stage model was still small, with 7% of the variation across studies due to heterogeneity (i.e., I2 = 7%). Analyses of drug-coated balloon and sensitivity analysis related to drug-eluting stent (Zilver® PTX®) specific mortality are reported in Table 3.
Table 3.
Crude Mortality by Cause of Death
| Cancer Related | Cardiovascular Related | Infectious Related | Pulmonary Related | Other Causes* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Paclitaxel N Deaths (% of subjects) | Control N Deaths (% of subjects) | Paclitaxel N Deaths (% of subjects) | Control N Deaths (% of subjects) | Paclitaxel N Deaths (% of subjects) | Control N Deaths (% of subjects) | Paclitaxel N Deaths (% of subjects) | Control N Deaths (% of subjects) | Paclitaxel N Deaths (% of subjects) | Control N Deaths (% of subjects) |
| All Studies | 57 (2.6%) | 22 (1.0%) | 64 (2.9%) | 33 (1.5%) | 12 (0.5%) | 7 (0.3%) | 14 (0.6%) | 2 (0.1%) | 41 (1.9%) | 19 (0.9%) |
| ILLUMENATE Pivotal | 5 (1.7%) | 3 (1.0%) | 7 (2.3%) | 10 (3.3%) | 2 (0.7%) | 0 (0%) | 2 (0.7%) | 0 (0%) | 6 (2.0%) | 1 (0.3%) |
| ILLUMENATE EU RCT | 14 (4.8%) | 3 (1.0%) | 11 (3.7%) | 1 (0.3%) | 5 (1.7%) | 1 (0.3%) | 0 (0%) | 0 (0%) | 1 (0.3%) | 3 (1.0%) |
| IN.PACT SFA I/II | 5 (1.5%) | 4 (1.2%) | 11 (3.3%) | 3 (0.9%) | 1 (0.3%) | 1 (0.3%) | 2 (0.6%) | 0 (0%) | 10 (3.0%) | 2 (0.6%) |
| IN.PACT Japan | 3 (3.0%) | 0 (0%) | 0 (0%) | 2 (2.0%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Levant 1 | 1 (1.0%) | 1 (1.0%) | 2 (2.0%) | 2 (2.0%) | 1 (1.0%) | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.0%) |
| Levant 2 | 17 (3.6%) | 5 (1.1%) | 18 (3.8%) | 5 (1.1%) | 2 (0.4%) | 3 (0.6%) | 3 (0.6%) | 0 (0%) | 15 (3.2%) | 4 (0.8%) |
| Lutonix Japan | 1 (0.9%) | 0 (0%) | 0 (0%) | 1 (0.9%) | 0 (0%) | 1 (0.9%) | 0 (0%) | 0 (0%) | 1 (0.9%) | 1 (0.9%) |
| Zilver PTX RCT | 11 (2.3%) | 6 (1.3%) | 15 (3.2%) | 9 (1.9%) | 0 (0%) | 0 (0%) | 7 (1.5%) | 2 (0.4%) | 8 (1.7%) | 7 (1.5%) |
Includes all other causes of death, including those with unknown relatedness.
Analysis of Dose Response Relation
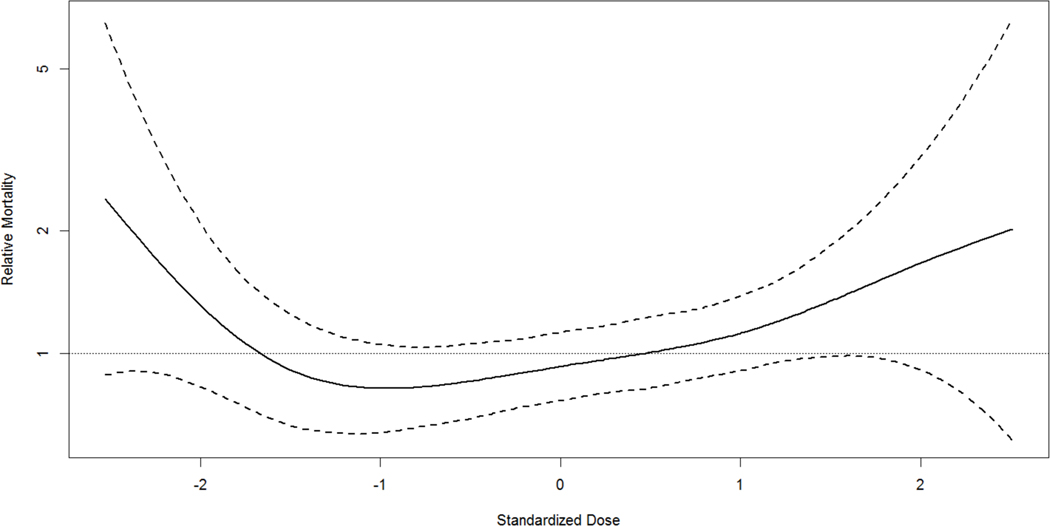
Exploratory analyses revealed no association of mortality risk and increasing drug dose10. Compared to non-paclitaxel exposed subjects, the HR was 1.30 (95% CI, 0.92 to 1.82) for the low exposure group, 1.23 (95% CI, 0.87 to 1.73) for the medium exposure group, and 1.41 (95% CI, 0.96 to 2.07) for the high exposure group showing that mortality risk did not increase with exposure (Figure 5).
Figure 5. Dose Response Spline Fit.
Values of relative mortality greater than 1 (horizontal line) indicate an increased risk relative to the mean standardized dose. Dashed lines are based on +/− 1.96 times the standard error.
Causes of Death
Causes of mortality are summarized in Table 3 based on simple unadjusted percentages. There was higher crude incidence of mortality for PTXD relative to control across all causes with no specific cause identified as particularly responsible for the overall increase.
Subgroup Analyses
Subgroup analyses were performed according to baseline variables of age, sex, race, smoking, body mass index (BMI), coronary artery disease (CAD), hypertension, hyperlipidemia, diabetes, renal insufficiency, history of myocardial infarction (MI), history of PCI, baseline medication use (antiplatelet, antithrombotic agents, statins, angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB), beta-blockers), Rutherford class, target limb ankle brachial index (ABI), number of target lesions, lesion type, location, length, percent stenosis, and minimal lumen and vessel diameter. None showed substantial evidence for treatment interaction (Figures S2-S4, all interaction p>0.05).
Discussion
This IPD meta-analysis demonstrated a 38% relative increase in risk of death in patients treated with PTXD for femoropopliteal PAD compared to controls and an estimated absolute risk increase of 4.6% over 5 year follow-up. Inclusion in the analysis of supplemental vital status data recovered after the primary analysis showed a 27% relative increased mortality risk. This observation was consistent across a wide range of pre-specified sensitivity analyses that made different assumptions and used different statistical models, including ITT and AT analyses. The estimate of increased mortality is smaller than previously reported from an aggregate meta-analysis3 that included 28 trials (4,663 patients) at 1 year, 12 trials (2,316 patients) at 2 years and 3 trials (863 patients) at 4 to 5 years of follow-up and reported a 93% increased relative risk and 6.6% increased absolute risk of all-cause mortality in patients exposed to PTXDs compared to controls at 4–5 years.
Our findings suggest selection bias is influential when using an aggregate data approach made worse by the limited number of long-term follow-up studies.15 Recent publications using other data sets provide detailed product-specific patient-level data but either combined a limited number of randomized studies with non-randomized observational cohort studies16 or reported considerable rates of missing vital status. 2, 17 These disparate study designs, mixed data sources, and variable ascertainment rates may explain differences in reported mortality outcomes. Our analysis minimized these limitations by using only patient-level data derived from randomized trials and analyzing recovered, previously unreported vital status data. Exclusion of two studies without patient-level data available may introduce bias, though this would be small given the studies modest size and duration of follow-up. Exclusion of studies with shorter follow-up may also introduce bias, however, this analysis sought the assessment of a longer-term outcome. The mortality hazard in our IPD meta-analysis has probative value and should be used to inform clinical practice.
Under a range of conservative assumptions, and a number of sensitivity analyses with missing data, the results remained consistent. Despite this, we cannot exclude the possibility that missing data affects importantly the current estimates of mortality risk. An effort was made to retrieve missing vital status information for participants in the trials, but 9.5% of the participants remained lost or withdrew without complete follow-up. Inclusion of previously unreported recovered vital status data analyses reduced the hazard ratio from 1.38 to 1.27; however, the reduction was modest relative to the width of the confidence intervals. The potential of a selection bias in the late data recovery must be acknowledged. The possibility that ascertainment bias may exert a disproportional influence on study conclusions was demonstrated in the Vascular Quality Initiative, a prospective multicenter registry of vascular and endovascular therapies, where patients treated with endovascular devices and subsequently lost to follow up had a 6-fold increase in mortality at one year.18
Despite the large sample size and number of deaths, the overall pattern in this cohort suggests increases in risk with PTXD for all reported causes of death. Therefore, any causal inferences need to be made with caution. Furthermore, we did not observe evidence for effect modification of the mortality risk across a number of patient subgroups, including Rutherford classification. As a final consideration, our drug dose-mortality analysis utilized patient level index procedure drug load data (total μg/mm2) and did not establish an association between increasing drug exposure and mortality through 5 years. Dose-mortality analysis is not protected by randomization, and while we adjusted for covariates, the potential for residual confounding cannot be ruled out.
In this regard, while it would be tempting to use preclinical data to more accurately predict the patient exposure index, such an approach would also introduce uncontrolled errors as the fraction of tissue transferred drug varies not only with the balloon coating type, but for a given coating can vary several-fold owing to differences in arterial morphology. This is supported by clinical data showing reduced efficacy of PTXD with the length and arc of arterial calcification19, likely owing to a combination of suboptimal apposition and diminished drug distribution properties20. Moreover, preclinical studies with fully apposed PTXD demonstrated that paclitaxel transfer affected by species. Thus, our classification of treatments using procedural nominal loads as an index are a recognized limitation of all current meta-analyses of PTXD, and point to the need for further research into the determinants of coating transfer from PTXD and similar technologies using sirolimus.
Nonetheless, our methodology is superior to a previous report3 that relied on summary data of PTXD dose, mean lesion lengths, reference vessel diameters and defined drug exposure as a function of the duration of study follow-up, the latter inducing potentially spurious correlation. This also assumes that paclitaxel tissue residence is time invariant, which is inconsistent with preclinical and clinical data showing a decline in tissue and blood content over time.21 Computational modeling of paclitaxel tissue uptake and distribution resulting from the dissolution of locally delivered coating particles predicts that soluble paclitaxel retention is mediated via high affinity binding to its pharmacological target (intracellular microtubules) limiting late systemic exposure.22 Indeed, these analyses raise questions regarding the plausibility of a direct relation between paclitaxel exposure and late mortality. Taken together, we have been unable to identify a mechanism of death. The lack of both a drug dose-mortality relation and a causal explanation for the mortality risk defines an area requiring further investigation. The increased all-cause mortality risk with PTXD in these analyses must be considered in context with contemporary literature of their use in other vascular beds and the use of paclitaxel in other disease states. Paclitaxel-eluting stents were originally used in the coronary arteries and fell out of favor on the basis of a small increase in late stent thrombosis. A substantial majority of the coronary paclitaxel stent literature23–27 shows no increase in all cause or cardiovascular mortality, although there is an exception that reported a modest increase in composite rate of cardiac death or myocardial infarction and stent thrombosis when compared to bare metal coronary stents in a smaller patient cohort (n=1,400). 28 Further, intravenous paclitaxel, formulated with cremophor in pregnant women with breast cancer in concentrations 50-fold higher than those used in the peripheral arteries, was safe for both patient and fetus.29 Though this chemotherapeutic modality differs from paclitaxel released from PTXD, the experience does underscore that there is currently no support for a residual pool of paclitaxel available after intravascular exposure to cause late mortality events, as paclitaxel is receptor bound. 30, 31 Preclinical reports provide additional insight into the metabolism of paclitaxel and its elution prolife. Schornl et al., reported nanomolar paclitaxel retention in arterial tissue out to 180 days after treatment with paclitaxel coated balloons32 results in picomolar concentrations of unbound PTX in the arterial wall22 and whose slow elution back into circulation has no biological relevance. Moreover, in vitro immersion of paclitaxel coated balloons in porcine plasma at body temperature suggest that any coating particles in free circulation will dissolve within hours33 to days and be eliminated from the body with a half life of 13–20 hours.34
Two large analyses of Medicare PAD patients offer additional insights into PTXD use and mortality. Secemsky et al. demonstrated no increase in mortality in combined paclitaxel DCB and DES patients compared to non-PTXDs (n = 16,560) at a median of 389 [IQR 277 – 507] days or for DES vs. BMS patients (n = 51,456) at a median follow-up of 2 [IQR 1.2 – 3.0] years. 35, 36 In a contemporary analysis of over 92,000 individuals in the German BARMER Health Insurance system, Freisinger et al. analyzed de-identified data from 23,137 patients who underwent an endovascular revascularization that included PTXDs from 2007 through 2017.37 A multivariable Cox regression analysis showed no increased mortality in patients receiving PTXDs compared to those receiving balloon angioplasty or bare metal stents over 11 year follow-up. While claims-based observational data suffer from uncontrolled residual confounding and selection bias, the size and completeness of vital status based analyses are valuable. Despite these contrary data from large population studies, safety in coronary trials, and established drug safety for its systemic use, the issue of risk versus benefit must be considered given the increased mortality we are reporting. Nevertheless, some patients may prefer gains in quality of life and reduced revascularization procedures even at the expense of potentially increased mortality risk.
We acknowledge several important limitations of this analysis. Overall, the dataset has a limited number of events to adequately evaluate an unplanned safety endpoint. Moreover, in acceptance with FDA guidance and agreement with industry collaborators, our analysis was restricted to patient level data sets of FDA approved, commercially available PTXDs with at least two-year follow-up as of March 2019. As such, any direct comparisons with other larger aggregate meta-analytic data sets3 is not possible. Additionally, the lack of patient comorbidity data may have hindered better ascertainment of the etiology of excess mortality. Additionally, the losses to follow-up and study withdrawal rates in the control and treatment arms of the RCTs evaluated in this IPD meta-analysis were 24% and 23%, respectively (Figure S5). Despite aggressive efforts at reducing patient loss, approximately 10% of patient remained lost to follow up. As such, the challenges addressed by this IPD meta-analysis offer an important lesson for future clinical trials of endovascular devices. Compared to another contemporary PAD trial of ticagrelor versus clopidogrel, loss to follow-up was 2% of the cohort over a median follow up of 30 months.38 Thus, future endovascular device trial designs must incorporate strategies and methods to maximize patient retention and facilitate long-term assessment and reporting of vital statistics. Such efforts should be undertaken in concert with added focus on preclinical, pharmacokinetic and computational models of novel agents.
In conclusion, this IPD meta-analysis demonstrated a 38% increased mortality risk associated with PTXD exposure using an ITT analysis. No association between drug dose and mortality was established. Loss to follow-up and study withdrawal rates in both treatment arms were high; inclusion of available recovered data further reduced the mortality risk.
Supplementary Material
Clinical Perspective.
What is new?
This comprehensive patient-level data meta-analysis derived from 8 randomized, controlled trials of FDA-approved paclitaxel-coated devices (paclitaxel balloons and stents) compared to balloon angioplasty observed a 4.6% absolute risk of increased mortality associated with paclitaxel-coated device use compared to balloon angioplasty at a median 4-year follow-up.
Significant lost to follow-up and withdrawal rates of 24% and 23% in the balloon angioplasty and paclitaxel cohorts, respectively, through 5-years were observed.
Recovery of lost vital status data reduced the observed paclitaxel device associated mortality rate.
No paclitaxel drug dose-mortality relationship was identified.
What are the Clinical Implications?
An aggregate meta-analysis of paclitaxel containing devices by Katsanos et. al., reported statistically significant increased late all-cause mortality at 2 and 5 years post treatment although no causal relationship was identified.
Freedom of target lesion revascularization and quality of life are improved with paclitaxel device use; as such, a discussion of the risk versus benefits of their use must be considered in the patient consent process.
To minimize selection bias, future endovascular device trial designs must incorporate strategies and methods to maximize patient retention and facilitate long-term assessment and reporting of clinical outcome and vital statistics.
Acknowledgments
VIVA Physicians (vivaphysicians.org) is a not-for-profit 401 c (3) organization dedicated to advancing the field of vascular medicine and interventions through education, research, advocacy and collaboration. The authors would like to thank the following individuals and companies for their support and transparency in facilitating access to de-identified data presented in this IPD meta-analysis: Drs. JD Meler and John DeFord, Becton Dickinson and Company; Aaron Lottes and Kian Olsen, Cook Medical Inc.; Mark Pacyna, Medtronic Inc.; Jonathan Batiller and Dr. Anthony Mullin, Philips Inc. We also wish to acknowledge Jennifer Mischke, Lisa Thackery, Tyson Rogers, Steven Ullery and Benjamin Brown from NAMSA and Christopher Ebbe from VIVA Physicians for their professionalism and commitment to the timely completion of this important work.
Sources of Funding
VIVA Physicians, Inc., San Jose, CA.
Non-standard Abbreviations and Acronyms:
- PTXD
Paclitaxel-containing Devices
Footnotes
Disclosures
Dr. Rocha-Singh is a consultant for Medtronic Inc., Alucent Biomedical, Philips, Pedra Technology and is a Board Member of VIVA Physicians, a 401 c(3) not for profit education and research organization. Dr. Jaff is a non-compensated advisor to Boston Scientific and is a compensated advisor to Abbott Vascular, Micell, Inc., Primacea, Medtronic, Biotronik, Philips, Sanofi, Silk Road Medical, Vactronix and Venarum. He is an equity investor in Efemoral, Embolitech, Gemini, Janacare, Inc., MC 10, PQ Bypass, Primacea, Sano V, Inc., and Vascular Therapies. Dr. Lyden is a non-compensated consultant to Philips and a consultant to BSC, Abbott, Endologix, Medtronic, Shockwave, and PQ Bypass and is a VIVA Board Member. Dr. Ansel is a consultant for Medtronic, Boston Scientific, Abbott Vascular, Surmodics, Reflow Medical, Phillips, and Veryan Inc. and receives royalties from Cook Medical; he a VIVA Board Member. Dr. Schneider is a member of the advisory board for Medtronic, Abbott, and Boston Scientific; has served as a consultant for Surmodics, Silk Road Medical, Medtronic, Cardinal, CSI, and Profusa and is a Chief Medical Officer for Intact Vascular and Cagent and is a Board Member of VIVA Physicians. Dr. Edelman discloses NIH grant GM49039, research funding from Abiomed, BSC, Edwards Life Sciences, Medtronic, consulting fees from Canon, MiCell, Peregrine, Tekla and is an equity investor in Autus, BioDevek and Panther. Dr.Tzafriri is an employee of CBSET, Lexington, MA. Dr. Misra discloses NIH grants HL098967, DK107870 and a Boehringer-Ingelheim Research Grant. Mr. Mullin is an employee of NASMA, Minneapolis, MN. Dr. Beckman reports consulting with Astra Zeneca, Bristol Myers Squibb, Amgen, Merck, Novo Nordisk, Sanofi, and Antidote Pharmaceutical. He serves on the DSMC for Bayer and Novartis. Dr. Duval was a paid statistical consultant to VIVA Physicians. Drs. Granada, Ioannidis and White have nothing to disclose.
References
- 1.Laird J, Schneider P, Jaff M, Brodmann M, Zeller T, Metzger D, Krishnan P, Scheinert D, Micari A, Wang H, et al. Long-term clinical effectiveness of a drug-coated balloon for the treatment of femoropopliteal lesions: 5-year outcomes from the IN.PACT ™SFA randomized trial. Circ Cardiovasc Interv. 2019; 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dake M, Ansel G, Jaff M, Ohki T, Saxon R, Smouse B, Machan L, Snyder S, O’Leary E, Raghed A, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver® PTX® randomized trial. Circulation. 2016; 133: 1472–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: A systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018; 7:e011245. doi: 10.1161/JAHA.118.011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration UPDATE: Treatment of Peripheral Arterial Disease with Paclitaxel-Coated Balloons and Paclitaxel-Eluting Stents Potentially Associated with Increased Mortality - Letter to Health Care Providers. https://www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm633614.htm. Published January 17, 2019. Accessed 20Aug2019.
- 5.US Food and Drug Administration Update: Treatment of peripheral arterial disease with paclitaxel-coated balloons and paclitaxel-eluting stents potentially associated with increased mortality—letter to health care providers. https://www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm633614.htm. Published March 15, 2019. Accessed 20Aug2019.
- 6.US Food and Drug Administration Update: Treatment of peripheral arterial disease with paclitaxel-coated balloons and paclitaxel-eluting stents potentially associated with increased mortality—letter to health care providers. https://www.fda.gov/MedicalDevices/Safety/LetterstoHealthCareProviders/ucm633614.htm. Published August 7, 2019. Accessed 20Aug2019.
- 7.Burke D, Ensor J, Riley R. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Statistics in Medicine. 2017; 36: 855–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall W, Wellner J. Confidence Bands for a Survival Curve for Censored Data. Biometrika. 1980; 67:1, 133–143. [Google Scholar]
- 9.Higgins J, Altman D, Gøtzsche P, Jüni P, Moher D, Oxman A, Savovic J, Schulz K, Weeks L, Sterne J. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. British Med J. 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eilers, Paul H. and Marx, Brian D. Flexible smoothing with B-splines and penalties. Statistical Science. 1996; 11: 89–121. [Google Scholar]
- 11.Stewart L, Clarke M, Rovers M, Riley R, Simmonds M, Stewart G, Tierney J, for the PRISMA-IPD Development Group. Preferred reporting items for a systematic review and meta-analysis of individual participant data: The PRISMA IPD statement. J. American Medical Association. 2015; 313: 1–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 12.Gouëffic Y, Sauguet A, Desgranges P, Feugier P, Rosset E, Ducasse E, Cardon A, Rinckenbach S, Pernes J, Commeau P, et al. The BATTLE Trial comparing bare metal to drug eluting stents for intermediate lesion length in the SFA. ZCT 02004951. Oral presentation at: LINC Congress, January 2016; Leipzig, Germany. [Google Scholar]
- 13.Fanelli F, Cannavale F, Corona M, Lucatelli A, Wledrk A, Salvatori K. The “DEBELLUM” – lower limb multilevel treatment with drug eluting balloon – randomized trial: 1-year follow-up results. J Cardiovasc Surgery. 2014; 55:207–216. [PubMed] [Google Scholar]
- 14.Rao S, Schoenfeld D. Survival Methods. Circulation. 2007; 115: 109–113. [DOI] [PubMed] [Google Scholar]
- 15.Juni P, Altman D, Egger M, et al. Systematic reviews in health care: Assessing the quality of controlled clinical trials. British Med J. 2001; 323: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider P, Laird J, Doros G, Gao S, Ansel G, Brodmann M, Micari A, Shishehbor M, Tepe G, Zeller T. Mortality not correlated with paclitaxel exposure: An independent patient-level meta-analysis of a drug-coated balloon. J Am Coll Cardiol. 2019; 73: 2550–2562. [DOI] [PubMed] [Google Scholar]
- 17.Correction to: Dake M, Ansel G, Jaff M, Ohki T, Saxon R, Smouse B, Machan L, Snyder S, O’Leary E, Raghed A, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation. 2019; 139: e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Judelson D, Goodney P, Bertges D. Loss to follow-up 1 year after lower extremity peripheral vascular intervention is associated with worse survival. Vas. Med. 2019; 24: 332–338. [DOI] [PubMed] [Google Scholar]
- 19.Elkhabaz A, Sarkar S, Dinh J, Simpson G, Taylor L. Variation in supersaturation and phase behavior of ezetimibe amorphous solid dispersions upon dissolution in different biorelevant media. Mol Pharm. 2018; 15: 193–206. [DOI] [PubMed] [Google Scholar]
- 20.Tzafriri A, Garcia-Polite F, Zani B, Stanley J, Muraj B, Knutson J, Kohler R, Markham P, Nikanorov A, Edelman E. Calcified plaque modification alters local drug delivery in the treatment of peripheral atherosclerosis. J Control Release. 2017: 264: p. 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan P. Faries K. Niazi A, Jain A, Sachar R, Bachinsky W, Cardenas J, Werner M, Brodmann M, Mustapha J, et al. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017; 136: 1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzafriri A, Parikh S, Edelman E. Taking paclitaxel coated balloons to a higher level: Predicting coating dissolution, tissue retention and dosing dynamics. J Controlled Release. 2019; 301: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarantini G, Facchin M, Capodanno D, Musumeci G, Saia F, Menozzi A, Meliga E, Mancone M, Lettieri C, Tamburino C. Paclitaxel versus sirolimus eluting stents in diabetic patients: does stent type and/or stent diameter matter?: long-term clinical outcome of 2,429-patient multicenter registry. Catheter Cardiovasc Interv. 2013; 81:80–89. [DOI] [PubMed] [Google Scholar]
- 24.Kereiakes D, Smits P, Kedhi E, Parise H, Fahy M, Serruys P, Stone G. Predictors of death or myocardial infarction, ischaemic-driven revascularisation, and major adverse cardiovascular events following everolimus-eluting or paclitaxel-eluting stent deployment: pooled analysis from the SPIRIT II, III, IV and COMPARE trials. EuroIntervention. 2011; 7:74–83. [DOI] [PubMed] [Google Scholar]
- 25.Simsek C, Magro M, Boersma E, Onuma Y, Nauta S, Gaspersz M, van der Giessen W, van Domburg R, Serruys P; Interventional Cardiologists of the Thoraxcenter. The unrestricted use of sirolimus- and paclitaxel-eluting stents results in better clinical outcomes during 6-year follow-up than bare-metal stents: an analysis of the RESEARCH (Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital) and T-SEARCH (Taxus-Stent Evaluated At Rotterdam Cardiology Hospital) registries. J Am Coll Cardiol Cardiovasc Interv. 2010; 3:1051–1058. [DOI] [PubMed] [Google Scholar]
- 26.Hermiller JB, Fergus T, Pierson W, Su X, Sood P, Sudhir K, Stone G. Clinical and angiographic comparison of everolimus-eluting and paclitaxel-eluting stents in small coronary arteries: a post hoc analysis of the SPIRIT III randomized trial. Am Heart J. 2009; 158:1005–1010. [DOI] [PubMed] [Google Scholar]
- 27.Stettler C, Wandel S, Allemann S, Kastrati A, Morice M, Schomig A, Pfisterer M, Stone G, Leon M, de Lezo J, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007; 370: 937–948. [DOI] [PubMed] [Google Scholar]
- 28.Stone G, Ellis S, Colombo A, Grube E, Popma J, Uchida T, Bleuit J, Dawkins K, Russell M. Long-term safety and efficacy of paclitaxel-eluting stents. Final 5-Year analysis from the Taxus clinical trial program. J Am Coll Cardiol Cardiovasc Interv. 2011; 4:530–542. [DOI] [PubMed] [Google Scholar]
- 29.Mir O, Berveiller P, Goffinet F, et al. Taxanes for breast cancer during pregnancy: a systematic review. Annals Oncology. 2010; 21:425–426. [DOI] [PubMed] [Google Scholar]
- 30.Tzafriri AR, Vukmirovic N, Kolachalama VB, Edelman E. Lesion complexity determines arterial drug distribution after local drug delivery. J Control Release. 2010; 142:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin A, Vukmirovic N, Hwang C, Edelman E. Specific binding to intra cellularproteins determines arterial transport properties for rapamycin and paclitaxel. PNAS. 2004; 101: 9463–9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schornl I, Malinoff H, Anderson S, Lecy C, Wang J, Giorgianni J, Papandreon G. The Lutonix drug-coated balloon: A novel drug delivery technology for the treatment of vascular disease. Advance Drug Delivery Reviews. 2017; 112:78–87 [DOI] [PubMed] [Google Scholar]
- 33.Granada J, Virmani R, Schulz-Jander D, Tunev S, Melder R. Rate of drug coating dissolution determines in-tissue drug retention and durability of biological efficacy. J Drug Deliv. 2019; 2019:9560592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Food and Drug Administration. FDA Executive Summary: Circulatory System Devices Panel Meeting June 19–20, 2019. Section 2.3.1, page 11; https://www.fda.gov/media/127698/download. Accessed January 17, 2020. [Google Scholar]
- 35.Secemsky EA; Kundi H; Weinberg I, Jaff M, Krawisz A, Parikh S, Beckman J, Mustapha J, Rosenfield K, Yeh R. Association of survival with femoropopliteal artery revascularization with drug-coated devices. JAMA Cardiol. 2019; 4: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Secemsky E, Kundi H, Weinberg I, Schermerhorn M, Beckman J, Parikh S, Jaff M, Mustapha J, Rosenfield K, Yeh R. Drug-eluting stent implantation and long-term survival following peripheral artery revascularization. J Am Coll Cardiol. 2019; 73: 2636–2642. [DOI] [PubMed] [Google Scholar]
- 37.Freisinger E, Koeppe J, Gerss J, Goerlich D, Malyar M, Marschall U, Faldum A, Reinecke H. Mortality after use of paclitaxel-based devices in the peripheral arteries: a real-world safety analysis. Eur Heart J. October 8, 2019; doi: 10.1093/eurheartj/ehz698. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiatt W, Fowkes G, Heizer G, Berger J, Baumgarter I, Held P, Katona B, Mahaffey K, Norgren L, Jones W, et al.Ticagrelor versus clopidogrel in symptomatic peripheral arterial disease. New Engl J Med. 2017; 376: 32–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.