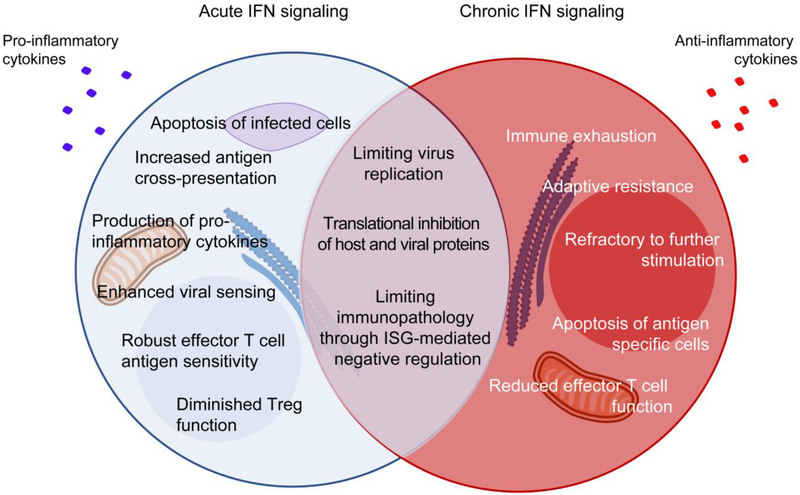
Figure 2: Chronic IFN-I signaling directly and indirectly induces immune activation and inflammation leading to an exhausted state.
Following infection, robust IFN-I signaling allows for strong antiviral responses characterized by inhibition of viral replication, apoptosis of infected cells, increased antigen cross-presentation, enhanced effector functions and overall control of infection. While chronic IFN-I signaling contributes towards controlling virus replication and limiting inflammation-induced immunopathology, it also leads to chronic immune stimulation and ultimately, immune dysfunction. The immunosuppressed or exhausted state resulting from chronic IFN-I signaling is characterized by several features including direct overexpression and accumulation of inhibitory factors (PDL1, IL-10, IDO, TRAIL, among others), and indirect effects due to chronic immune activation it drives (e.g., PD1, CD38, Tim3, CD38, among others). The ultimate outcome of these events is defective cytokine production, reduced metabolic fitness, adaptive resistance to therapy, failure to respond to further stimulation, contraction of antigen-specific cells, overall contributing to promote viral persistence.
PD-1 – programmed cell death-1; PDL1 – PD-1 ligand; Tim-3 – T-cell immunoglobulin and mucin-domain containing-3; IDO – indoleamine-2,3 dioxygenase; TRAIL – tumour-necrosis factor (TNF)-related apoptosis-inducing ligand.