Abstract
Impaired orthostatic blood pressure (BP) stabilization is prevalent in patients with chronic kidney disease (CKD) and is associated with adverse outcomes. We aimed to test the hypothesis that reduced hemoglobin is an important contributor to orthostatic intolerance in CKD in the present study. This study included 262 patients with non‐dialysis‐dependent CKD. Seated and standing BP was measured, and orthostatic BP reduction was calculated for both systolic BP (∆ SBP) and diastolic BP (∆ DBP). The association between orthostatic BP reduction and hemoglobin was determined by multiple linear regression models. We also performed mediation analysis to test to what extent the effect of renal dysfunction on impaired orthostatic BP stabilization can be explained by reduced hemoglobin. The mean age of the patients was 57.7 (±14.5) years, and 61.5% were male. Both ∆ SBP and ∆ DBP correlated negatively with estimated glomerular filtration rate (eGFR). With adjustment for age and sex, hemoglobin level was negatively associated with ∆ SBP (β = −1.4, SE = 0.4, P < .001) and ∆ DBP (β = −0.6, SE = 0.2, P = .009). The associations remained significant with further adjustment for additional covariates. When eGFR was introduced as a covariate, it did not eliminate the significance (both P < .05). The associations remained essentially unchanged in a sensitivity analysis excluding those with concurrent erythropoietin use. Mediation analysis demonstrated that reduced hemoglobin accounted for 35.4% (P = .004) of the effect of eGFR on ∆ SBP and 47.7% (P = .032) on ∆ DBP. Our study suggests that reduced hemoglobin is a potentially important contributor to the development of orthostatic hypotension in CKD.
Keywords: anemia, blood pressure, CKD, orthostatic hypotension, renal dysfunction
1. INTRODUCTION
Impaired cardiovascular health is an important sequela of chronic kidney disease (CKD).1 Various traditional and nontraditional cardiovascular risk factors that accumulate during the development and progression of CKD compromise normal cardiovascular function.2 As a result, patients with CKD are prone to developing a series of conditions due to disturbed cardiovascular homeostasis, such as impaired orthostatic blood pressure (BP) stabilization. Recent data from the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that worse renal function was independently associated with greater reductions in systolic BP on standing.3 This impaired orthostatic BP stabilization has been associated with a number of adverse outcomes (eg, fall, syncope, cardiovascular events, and all‐cause mortality) in different study populations, including those with renal dysfunction.4, 5, 6, 7 It is therefore important to identify those at risk for this condition and to understand its underlying pathophysiologic mechanisms in CKD. However, there is a paucity of research exploring the risk factors for impaired orthostatic BP stabilization, especially in the context of CKD.
It was noted in previous studies that some patients with orthostatic hypotension are anemic and a pilot trial demonstrated that correction of anemia with erythropoietin (EPO) restores orthostatic homeostasis.8, 9 Anemia is a prominent feature of renal failure, but its association with orthostatic intolerance in CKD has not yet been studied. Given the aforementioned data, we hypothesized that reduced hemoglobin is an important contributor to CKD‐associated impaired orthostatic BP stabilization. Here, we test our hypothesis in a group of non‐dialysis‐dependent CKD patients.
2. METHODS
2.1. Study population
All participants were recruited from the Center for Kidney Disease of the Second Affiliated Hospital of Nanjing Medical University. Patients with a clinical diagnosis of CKD and aged ≥18 years referring to our center were invited to participate in the study. We excluded those on hemodialysis or peritoneal dialysis or with a previous history of renal transplantation. Patients with acute infection or any other condition that were clinically unstable were not included until they recovered. All subjects provided written informed consent before enrollment. The study protocol was approved by the Institutional Ethics Committee of the Second Affiliated Hospital of Nanjing Medical University.
2.2. BP measurement
BPs were measured in the morning (usually between 8:00 am and 9:00 am) in a quiet test room using an automated oscillometric device (Omron HEM‐7130; Omron Healthcare Co. Ltd). Patients were instructed to restrain from meals, coffee or tea, and alcohol before measurement. Scheduled antihypertensive medications and nitrates were put off until after the study procedures were completed. This meant that all relevant medications were generally discontinued for at least 12 hours before the test. After at least 10 minutes of rest, seated BP was measured thrice with a 1‐minute interval between measurements. Then, the patients were asked to stand up immediately, and three standing BPs were determined at 1‐minute intervals. Any symptom of orthostatic hypotension (eg, dizziness) reported by the participants while standing was also recorded. Participants were allowed to sit or lie down if they felt they could not keep standing during the process.
Seated BPs were calculated as the mean of the second and the third measurements. Orthostatic BP reductions were calculated as seated BPs minus the minimum of the three standing BPs and are denoted as ∆ SBP (orthostatic systolic BP reduction) or ∆ DBP (orthostatic diastolic BP reduction) hereafter. Orthostatic hypotension was defined as ∆ SBP ≥ 20 mm Hg or ∆ DBP ≥ 10 mm Hg.10
2.3. Pulse wave velocity
Carotid‐femoral pulse wave velocity (cfPWV) was determined using the Complior Analyzer device (Artech Medical) as described previously.11 Briefly, a highly experienced technician (5 years with over 2000 measurements) performed the measurement and placed the probes in a place with a palpable pulse of the carotid and femoral artery. The transit time was averaged over ten consecutive recordings using the intersecting tangent algorithm as recommended.12 Carotid‐femoral distance was measured manually and multiplied by 0.8 for further calculation. The cfPWV was calculated as the distance divided by the transit time.
2.4. Laboratory tests and other information
Fasting blood was drawn and sent to the Laboratory Department of our hospital for routine tests, including blood cell counts, serum albumin, lipids, calcium, phosphorus, and creatinine. The estimated glomerular filtration rate (eGFR) was calculated according to the CKD‐EPI formula.13 Patients were interviewed by research staff to obtain their general demographic and medical information. When necessary, medical records were also used for data acquisition or validation.
2.5. Statistical analysis
For descriptive analysis, mean ± SD or median (IQR) and frequency (%) are presented as appropriate. Comparisons between two groups were conducted using Student's t test, the chi‐squared test, or the Mann‐Whitney U test, as appropriate. The association between orthostatic BP reduction and renal function (eGFR)/hemoglobin was analyzed using Pearson's correlation analysis. To explore the association between orthostatic BP stabilization and reduced hemoglobin, we performed general linear regression analysis using ∆ SBP or ∆ DBP as the dependent variable. We built three models with different levels of adjustment for potential confounders. The determination of the confounders was based either on their correlation with orthostatic BP reduction in our dataset or on clinical consideration. In the first model (Model 1), we made basic adjustments for age and sex. In Model 2, additional adjustment was made for body mass index, current smoker, diabetes, previous cardiovascular disease, use of antihypertensive medications, statins, systolic blood pressure, albumin, and cfPWV. In the last model (Model 3), we included eGFR to test whether the association between hemoglobin and orthostatic BP reduction was independent of renal function. Several previous studies have demonstrated that the use of erythropoietin (EPO) to correct anemia can result in the alleviation of impaired orthostatic stabilization. Therefore, we performed a sensitivity analysis excluding those who were on concurrent EPO treatment. We also evaluated the association between eGFR and orthostatic BP reduction using a similar approach. All estimates for the association were calculated per 10 g/L increase in hemoglobin or per 10 mL/min/1.73 m2 increase in eGFR. To explore to what extent the effect of renal dysfunction on impaired orthostatic BP stabilization could be explained by reduced hemoglobin, we performed mediation analyses using hemoglobin as the mediator, eGFR as the predictor and ∆ SBP and ∆ DBP as the outcome variables. Age, sex, smoking status, and diabetes were treated as confounders in the mediation analyses. Other factors, including blood pressure, cfPWV, and albumin, were considered to contribute to the overall effect of renal dysfunction on impaired orthostatic BP stabilization and were not included in the first model but were included in an additional model. The bootstrapping approach was used to determine the significance of the mediation effect (simulations = 1000). All statistical analyses were performed using R software, version 3.4.3 (R Foundation for Statistical Computing, www.R-project.org). A two‐tailed P value of .05 was considered statistically significant.
3. RESULTS
The study included a total of 262 subjects. All patients underwent orthostatic blood pressure measurement. Seven of them reported feeling mildly lightheaded after standing which resolved automatically, and the measurements were therefore not discontinued for them. Standing BP was measured only once for one subject and twice for another due to their intolerance of the procedure, with overt symptoms of orthostatic hypotension (feeling weak, fatigue, and excessive sweating). Since their ∆ SBP calculated from existing standing BPs had already met the diagnostic criteria of orthostatic hypotension, they were not excluded from the analysis.
Table 1 presents the general characteristics of all the study subjects. The mean age was 57.7 (±14.5) years, and 61.5% were male. Glomerulonephritis (33.2%) and diabetic nephropathy (31.3%) were the leading causes of CKD in this population. The average eGFR was 50.8 (±33.4) mL/min/1.73 m2, and the mean hemoglobin level was 117.4 (±24.9) g/L. The median ∆ SBP and ∆ DBP were 5.5 (0‐12.0) and −0.5 (−4.0 to 3.4) mm Hg, respectively. A total of 42 subjects met the definition of orthostatic hypotension in our analysis, corresponding to a prevalence of 16.0% in this population. Patients with orthostatic hypotension were more likely to be male and diabetic, and they had lower levels of eGFR and hemoglobin. We also present the ∆BP at each standing measurement in Table S1.
Table 1.
General characteristics of the patients
| All | Orthostatic hypotension | |||
|---|---|---|---|---|
| No (N = 220) | Yes (N = 42) | P | ||
| Age, y | 57.7 ± 14.5 | 57.2 ± 14.8 | 60.8 ± 12.7 | .14 |
| Male | 161 (61.5%) | 141 (64.1%) | 20 (47.6%) | .044 |
| BMI, kg/m2 | 25.9 ± 4.1 | 25.9 ± 4.1 | 25.7 ± 4.3 | .743 |
| Current smoker | 74 (28.2%) | 66 (30.0%) | 8 (19.0%) | .149 |
| Etiology | ||||
| Diabetic nephropathy | 82 (31.3%) | 55 (25.0%) | 27 (64.3%) | <.001 |
| Hypertensive nephropathy | 23 (8.8%) | 23 (10.5%) | 0 (0.0%) | |
| Glomerulonephritis | 87 (33.2%) | 80 (36.4%) | 7 (16.7%) | |
| Others | 25 (9.5%) | 23 (10.5%) | 2(4.8%) | |
| Unknown | 45 (17.2%) | 39 (17.7%) | 6 (14.3%) | |
| Diabetes mellitus | 120 (45.8%) | 89 (40.5%) | 31 (73.8%) | <.001 |
| Previous CVD | 64 (24.4%) | 52 (23.6%) | 12 (28.6%) | .495 |
| Use of antihypertensives | 211 (80.5%) | 176 (80.0%) | 35 (83.3%) | .617 |
| Total number | 1.5 ± 1.1 | 1.5 ± 1.1 | 1.6 ± 1.0 | .511 |
| Diuretics | 24 (9.2%) | 21 (9.5%) | 3 (7.1%) | .776 |
| CCB | 149 (56.9%) | 118 (53.6%) | 31 (73.8%) | .016 |
| Beta blocker | 74 (28.2%) | 63 (28.6%) | 11 (26.2%) | .747 |
| ACEI/ARB | 121 (46.2%) | 105 (47.7%) | 16 (38.1%) | .251 |
| Other types | 18 (6.9%) | 13 (5.9%) | 5 (11.9%) | .180 |
| Statins | 109 (41.6%) | 89 (40.5%) | 20 (47.6%) | .388 |
| Use of EPO | 50 (19.1%) | 38 (17.3%) | 12 (28.6%) | .088 |
| eGFR, mL/min/1.73 m2 | 50.8 ± 33.4 | 53.5 ± 34.0 | 36.6 ± 25.9 | .002 |
| Hemoglobin, g/L | 117.4 ± 24.9 | 119.3 ± 25.2 | 107.6 ± 20.7 | .005 |
| Albumin, g/L | 36.5 ± 7.3 | 36.9 ± 7.3 | 34.7 ± 7.0 | .075 |
| Total cholesterol, mmol/L | 4.89 ± 1.75 | 4.80 ± 1.73 | 5.36 ± 1.81 | .059 |
| Triglycerides, mmol/L | 1.69 (1.11 to 2.55) | 1.69 (1.11 to 2.56) | 1.65 (1.12 to 2.45) | .800 |
| HDL‐C, mmol/L | 1.16 ± 0.43 | 1.15 ± 0.44 | 1.19 ± 0.36 | .661 |
| LDL‐C, mmol/L | 3.04 ± 1.36 | 2.98 ± 1.35 | 3.33 ± 1.35 | .129 |
| Calcium, mmol/L | 2.13 ± 0.17 | 2.13 ± 0.17 | 2.14 ± 0.17 | .785 |
| Phosphorus, mmol/L | 1.17 ± 0.25 | 1.15 ± 0.25 | 1.23 ± 0.22 | .052 |
| cfPWV, m/s | 10.5 ± 3.5 | 10.4 ± 3.6 | 11.3 ± 3.3 | .131 |
| Seated SBP, mm Hg | 136.3 ± 20.3 | 134.6 ± 19.2 | 145.0 ± 23.7 | .002 |
| Seated DBP, mm Hg | 84.1 ± 11.3 | 84.3 ± 11.0 | 83.3 ± 12.8 | .627 |
| Seated heart rate, bpm | 74.0 ± 12.4 | 74.4 ± 12.8 | 72.0 ± 9.9 | .249 |
| 1st standing SBP, mm Hg | 133.1 ± 20.4 | 134.1 ± 19.3 | 127.7 ± 24.8 | .063 |
| 1st standing DBP, mm Hg | 85.8 ± 12.1 | 87.5 ± 11.4 | 77.3 ± 11.9 | <.001 |
| 2nd standing SBP, mm Hg | 133.9 ± 21.6 | 135.1 ± 20.3 | 127.3 ± 27.1 | .033 |
| 2nd standing DBP, mm Hg | 86.4 ± 12.4 | 88.1 ± 11.5 | 77.3 ± 13.2 | <.001 |
| 3rd standing SBP, mm Hg | 133.5 ± 21.7 | 134.8 ± 20.5 | 126.3 ± 26.6 | .023 |
| 3rd standing DBP, mm Hg | 86.7 ± 12.7 | 88.5 ± 11.6 | 76.7 ± 14.0 | <.001 |
| ∆ SBP, mm Hg | 5.5 (0 to 12.0) | 4.25 (−1.13 to 8.50) | 23.8 (21.0 to 28.6) | <.001 |
| ∆ DBP, mm Hg | ‐0.5 (−4.0 to 3.4) | ‐1.0 (−4.5 to 1.0) | 9.5 (5.5 to 12.0) | <.001 |
Abbreviations: ∆ DBP, orthostatic diastolic blood pressure reduction; ∆ SBP, orthostatic systolic blood pressure reduction; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blockers; BMI, body mass index; CCB, calcium channel blockers; cfPWV, carotid‐femoral pulse wave velocity; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EPO, erythropoietin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure.
The relationship between orthostatic BP reduction and eGFR and hemoglobin is shown in Figure 1. Both orthostatic systolic and diastolic BP reductions were exaggerated with declined renal function (∆ SBP: r = −.31, P < .001; ∆ DBP: r = −.20, P = .001). A similar correlation was noted between orthostatic BP reduction and hemoglobin (∆ SBP: r = −.33, P < .001; ∆ DBP: r = −.23, P < .001).
Figure 1.
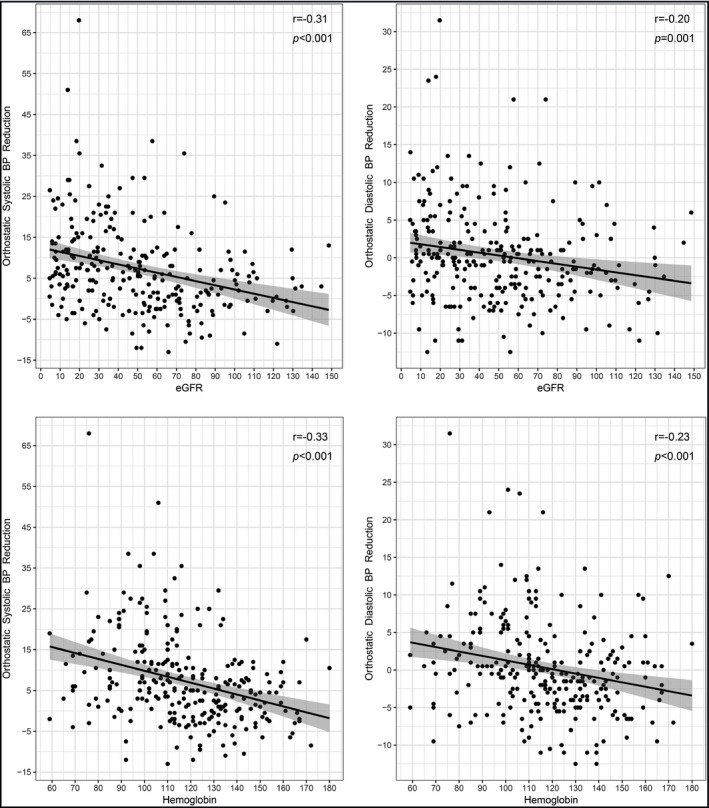
Correlations of orthostatic blood pressure reductions with estimated glomerular filtration rate and hemoglobin
We first analyzed whether eGFR was an independent determinant of orthostatic BP reduction using linear regression models. As shown in Table S2, eGFR was independently associated with ∆ SBP/∆ DBP in both the basic (age‐ and sex‐adjusted) model and the extensively adjusted model (all P ≤ .007). We then analyzed the associations between hemoglobin level and orthostatic BP reductions, and the results are presented in Table 2. In the basic model (Model 1) with adjustment for age and sex, hemoglobin level was negatively associated with ∆ SBP (B = −1.4, SE = 0.4, P < .001) and ∆ DBP (B = −0.6, SE = 0.2, P = .009). The associations remained significant with extensive adjustment in Model 2, and the effect sizes of the associations were relatively stable after further adjustment (∆ SBP: B = −1.2, SE = 0.4, P = .002; ∆ DBP: B = −0.6, SE = 0.2, P = .015). Moreover, when eGFR was included as a covariate, the associations were still significant for both ∆ SBP and ∆ DBP, although a marginal P value for ∆ DBP was noted (∆ SBP: B = −0.9, SE = 0.4, P = .021; ∆ DBP: B = −0.5, SE = 0.2, P = .045), while the estimates for eGFR were significant for ∆ SBP (B = −0.6, SE = 0.2, P = .013) but not for ∆ DBP (B = −0.2, SE = 0.2, P = .144).
Table 2.
Association between hemoglobin level and orthostatic blood pressure change
| ∆ SBP | ∆ DBP | |||||
|---|---|---|---|---|---|---|
| B a | 95% CI | P | B a | 95% CI | P | |
| Model 1 | −1.4 | 0.3 | <.001 | −0.6 | 0.2 | <.001 |
| Model 2 | −1.1 | 0.3 | <.001 | −0.6 | 0.2 | .003 |
| Model 3 | −0.7 | 0.3 | .035 | −0.4 | 0.2 | .049 |
Model 1: adjusted for age and sex;
Model 2: Model 1 + body mass index, current smoker, diabetes, previous cardiovascular disease, use of antihypertensive medications, statins, systolic blood pressure, albumin, and carotid‐femoral pulse wave velocity.
Model 3: Model 2 + eGFR.
Abbreviations: ∆ DBP, orthostatic diastolic blood pressure reduction; ∆ SBP, orthostatic systolic blood pressure reduction; eGFR, estimated glomerular filtration rate.
All estimates were calculated per 10 g/L increase in hemoglobin.
We further performed a sensitivity analysis of the association in patients without concurrent EPO use. After excluding those on EPO (n = 50), 212 subjects remained for this sensitivity analysis (Table 3). The associations between hemoglobin level and orthostatic BP reductions remained essentially unchanged in these subjects in all three models. The association between hemoglobin and orthostatic systolic BP was then evaluated among patients with CKD due to diabetes and among patients with CKD due to other etiologies (Table S3). The association was significant in all models, except for Model 3 (eGFR included) in patients with CKD due to diabetes. This loss of significance may be due to the limited power of the small sample size in this subgroup (N = 82).
Table 3.
Association between hemoglobin level and orthostatic blood pressure change in patients not using erythropoietin
| ∆ SBP | ∆ DBP | |||||
|---|---|---|---|---|---|---|
| B a | SE | P | Ba | SE | P | |
| Model 1 | −1.4 | 0.4 | <.001 | −0.6 | 0.2 | .009 |
| Model 2 | −1.2 | 0.4 | .002 | −0.6 | 0.2 | .015 |
| Model 3 | −0.9 | 0.4 | .021 | −0.5 | 0.2 | .045 |
Model 1: adjusted for age and sex;
Model 2: Model 1 + body mass index, current smoker, diabetes, previous cardiovascular disease, use of antihypertensive medications, statins, systolic blood pressure, albumin, and carotid‐femoral pulse wave velocity.
Model 3: Model 2 + eGFR.
Abbreviations: ∆ DBP, orthostatic diastolic blood pressure reduction; ∆ SBP, orthostatic systolic blood pressure reduction; eGFR, estimated glomerular filtration rate.
All estimates were calculated per 10 g/L increase in hemoglobin.
To quantify the contribution of reduced hemoglobin to the effect of renal dysfunction on orthostatic BP stabilization, we then performed a mediation analysis. A diagram illustrating the associations underlying the mediation effect is presented in Figure S1. The results, as shown in Table 4, demonstrated that the effects of renal dysfunction (eGFR) on ∆ SBP and ∆ DBP were both significantly mediated by hemoglobin level, with reduced hemoglobin accounting for 35.4% (P = .004) of the effect on ∆ SBP and 47.7% (P = .032) of the effect on ∆ DBP. When blood pressure, cfPWV, and albumin were included in Model 2 as confounding variables, the mediation effects remained significant (∆ SBP: 34.8%, P = .012; ∆ DBP: 70.2%, P = .042).
Table 4.
Hemoglobin mediates the association of eGFR with orthostatic blood pressure change
| ∆ SBP | ∆ DBP | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | |
| Model 1 | ||||||
| ACME | −0.03 | −0.06 to −0.01 | .004 | −0.02 | −0.03 to 0.00 | .022 |
| ADE | −0.06 | −0.10 to −0.02 | <.001 | −0.02 | −0.04 to 0.01 | .178 |
| Total effect | −0.09 | −0.13 to −0.05 | <.001 | −0.03 | −0.06 to −0.01 | .010 |
| Proportion mediated | 35.4% | 12.0 to 65.0% | .004 | 47.7% | 5.1 to 155.0% | .032 |
| Model 2 | ||||||
| ACME | −0.03 | −0.06 to −0.01 | .012 | −0.02 | −0.04 to −0.01 | .010 |
| ADE | −0.06 | −0.10 to −0.02 | <.001 | −0.01 | −0.04 to 0.02 | .528 |
| Total effect | −0.09 | −0.13 to −0.05 | <.001 | −0.03 | −0.05 to 0.0 | .032 |
| Proportion mediated | 34.8% | 7.8 to 76.0% | .012 | 70.2% | 3.9 to 336.0% | .042 |
Abbreviations: ∆ DBP, orthostatic diastolic blood pressure reduction; ∆ SBP, orthostatic systolic blood pressure reduction; ACME, average causal mediation effects; ADE, average direct effect; eGFR, estimated glomerular filtration rate.
Model 1: adjusted for age, sex, smoker, and diabetes;
Model 2: Model 1 + systolic (for ∆ SBP)/diastolic (for ∆ DBP) blood pressure, carotid‐femoral pulse wave velocity, and albumin.
4. DISCUSSION
In the present study, we investigated the association of reduced hemoglobin with impaired orthostatic BP stabilization in CKD patients. Our results showed that reduced hemoglobin level in these patients was independently associated with more pronounced orthostatic BP reduction. Mediation analysis indicated that reduced hemoglobin accounted for over one‐third of the total effect of renal dysfunction on orthostatic BP stabilization. Therefore, our study suggests the important contribution role of anemia to the development of orthostatic hypotension in CKD.
As shown in our study, impaired orthostatic BP stabilization worsens as renal function declines. The effect size of this association was significant: every 10 mL/min/1.73 m2 decrease in eGFR was associated with an ≈1/0.4 mm Hg increase in the orthostatic SBP/DBP reduction. This association is in line with findings from previous large‐scale community‐based studies.14, 15 Using a widely adopted diagnostic criteria, we identified that 16.0% of the subjects had orthostatic hypotension. The markedly increased risk of orthostatic hypotension in patients with renal failure has been noted by previous investigations.3, 4, 16 In fact, risk factors for orthostatic hypotension, including diabetes, aging, and hypertension, are known to be highly prevalent in patients with CKD. However, there is a paucity of data on other pathologic factors that might contribute to orthostatic intolerance in the context of CKD other than the aforementioned traditional risk factors. The present study highlights the particular role of anemia in this CKD sequela, which might help to understand the underlying pathophysiologic mechanism and identify potential targets for intervention.
Anemia is an intrinsic characteristic of renal dysfunction. The kidney is the major producer of erythropoietin, which in turn stimulates red blood cell production in the bone marrow and hence maintains normal red blood cell turnover and a constant hemoglobin level. Anemia occurs due to insufficient EPO production when kidneys are diseased. It develops at the early stages of CKD and worsens as CKD progresses. In our study, most of the study subjects were anemic with an average hemoglobin level of 117.4 g/L. This diminished hemoglobin level was significantly associated with impaired orthostatic BP stabilization in these patients, independent of potential confounders, with every 10 g/L decrease in hemoglobin associated with a 0.7/0.4 increase in the orthostatic SBP/DBP reduction. More importantly, this finding remains significant even after adjustment for eGFR, indicating that the association is not just due to the fact that reduced hemoglobin is a proxy of CKD severity, but rather reflects the direct effect of reduced hemoglobin on orthostatic BP regulation.
There are several possible pathophysiologic pathways in which reduced hemoglobin could exert an effect on orthostatic BP regulation. First, hemoglobin can sequester and inactivate nitric oxide (NO), and this inactivation is reduced in the anemic state.17, 18 It has also been shown that anemia can induce renal and vascular NO synthase upregulation and increase NO production.19 Hence, tissue NO level is increased in response to reduced hemoglobin, which will result in peripheral vasodilation and lower systemic vascular resistance. In view of the fact that peripheral vasoconstriction is critical for systemic compensation for postural BP reduction,20 anemia can thus induce orthostatic hypotension. Second, the viscosity of the blood, which is largely dependent on the concentration of red blood cells,21 is reduced in anemia and this reduced blood viscosity will decrease peripheral vascular resistance as well. Third, anemia can result in a significant increase in cardiac output and an impaired cardiac reserve, which will limit cardiac compensation in certain situations such as exercise and orthostatic BP maintenance. Last, anemia might also impair the normal orthostatic BP regulation ability by exerting a negative effect on the autonomic nervous system that leads to autonomic dysfunction.22, 23
The causative effect of reduced hemoglobin on impaired orthostatic BP stabilization is supported by previous intervention studies with EPO. In an early milestone study, Hoeldtke et al9 treated eight orthostatic hypotensive patients with EPO and found that the treatment markedly alleviated postural BP reduction with increased hematocrit. Their finding was confirmed by several subsequent studies.24, 25, 26 It has to be pointed out that these studies are limited to a very small number of patients with heterogeneous etiologies, and there is a paucity of investigations into the effect of EPO on orthostatic hypotension in CKD patients, although EPO has been routinely used for anemia treatment in these patients. Notably, EPO has been shown to be vascularly active and can increase BP in addition to its erythropoietic effect.27, 28 It is still unclear whether the effect of EPO on orthostatic BP regulation is merely due to its effect of anemia correction or involves other direct effects on the vascular system as well. In our analysis, we demonstrated that the associations between hemoglobin level and orthostatic BP reductions remained significant in patients without concurrent EPO use, suggesting that this association was not due to the confounding effect of EPO treatment.
Our results, if confirmed, have potentially important clinical implications. As mentioned above, orthostatic hypotension is prevalent in patients with renal failure. It has been proven to be an independent predictor for mortality in those on maintenance hemodialysis as well as in the general population.6, 7, 29, 30 In addition to its association with outcome, it can also result in fall, fracture, and syncope, which will compromise patients' health and quality of life. Moreover, we and others have demonstrated that impaired orthostatic BP stabilization is associated with cognitive impairment in dialysis patients and in the elderly, respectively.31, 32 In light of these detrimental effects of orthostatic intolerance, our findings would inform clinicians to specifically screen for this condition in CKD patients with severe anemia. In addition, although EPO has been routinely used clinically for anemia correction in CKD, it should be checked whether the use of EPO is sufficient for restoring orthostatic homeostasis in these patients, especially given that current guideline recommends a target hemoglobin level for EPO treatment below general diagnosis criteria of anemia.33 Furthermore, our results may also provide insight into the pathogenesis of increased risk of cardiovascular events in CKD. For example, it is known that CKD patients suffer a higher risk of stroke and this increased risk is more pronounced in those with a combination of CKD and anemia.34 Since orthostatic hypotension is a proven risk factor for stroke,20, 35 it might be the pathophysiologic explanation for this exaggerated risk.
Our study has several limitations. First, due to the cross‐sectional observational design, causal inference of the associations found in the present study cannot be made. Second, the hemoglobin level was measured only once in our study while it is actually dynamic especially when EPO is used. However, this was partly addressed as we performed a sensitivity analysis excluding those on EPO treatment and confirmed the association in those without concurrent EPO use. Third, participants who were included in this study might be healthier than subjects who rejected the invitation to participate and, hence, the study group may not be a good reflection of the general CKD population, which might limit the generalizability of the results. Finally, although we tried to adjust for confounder extensively, there might still be some residual confounding effects as well as potential confounding factors not measured in the current study.
In conclusion, we found that reduced hemoglobin was independently associated with impaired orthostatic BP stabilization in a group of non‐dialysis‐dependent CKD patients. Our mediation analysis suggests that reduced hemoglobin contributes to a significant part of orthostatic BP reduction in renal failure. These results provide further insight into the pathophysiologic mechanism underlying impaired orthostatic homeostasis in CKD and warrant physicians' screening for orthostatic hypotension in those with combined CKD and anemia.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Wenjin Liu performed the analysis and drafted the manuscript; Lulu Wang and Xiaoqin Huang collected the data and contributed to the analysis; Weichun He contributed to the organization of the study and revised the manuscript; and Zongwei Song and Junwei Yang generated the idea and approved the final manuscript.
Supporting information
Liu W, Wang L, Huang X, He W, Song Z, Yang J. Impaired orthostatic blood pressure stabilization and reduced hemoglobin in chronic kidney disease. J Clin Hypertens. 2019;21:1317–1324. 10.1111/jch.13658
Liu and Wang contributed equally.
Funding information
This work was supported by a grant to Dr Junwei Yang from Jiangsu Science and Technology Department (BE2017762).
Contributor Information
Zongwei Song, Email: songzongwei@njmu.edu.cn.
Junwei Yang, Email: jwyang@njmu.edu.cn.
REFERENCES
- 1. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108:2154‐2169. [DOI] [PubMed] [Google Scholar]
- 2. Rucker D, Tonelli M. Cardiovascular risk and management in chronic kidney disease. Nat Rev Nephrol. 2009;5:287. [DOI] [PubMed] [Google Scholar]
- 3. Townsend RR, Chang TI, Cohen DL, et al. Orthostatic changes in systolic blood pressure among sprint participants at baseline. J Am Soc Hypertens. 2016;10:847‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu JS, Wu NH, Lu FH, Chang CJ. Factors associated with orthostatic hypotension in the chinese population in taiwan. Am J Hypertens. 1996;9:999‐1005. [DOI] [PubMed] [Google Scholar]
- 5. Fleg JL, Evans GW, Margolis KL, et al. Orthostatic hypotension in the accord (action to control cardiovascular risk in diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension. 2016;68:888‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sasaki O, Nakahama H, Nakamura S, et al. Orthostatic hypotension at the introductory phase of haemodialysis predicts all‐cause mortality. Nephrol Dial Transplant. 2005;20:377‐381. [DOI] [PubMed] [Google Scholar]
- 7. Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis‐associated hypotension as an independent risk factor for two‐year mortality in hemodialysis patients. Kidney Int. 2004;66:1212‐1220. [DOI] [PubMed] [Google Scholar]
- 8. Chokroverty S, Barron KD, Katz FH, Del Greco F, Sharp JT. The syndrome of primary orthostatic hypotension. Brain. 1969;92:743‐768. [DOI] [PubMed] [Google Scholar]
- 9. Hoeldtke RD, Streeten DH. Treatment of orthostatic hypotension with erythropoietin. N Engl J Med. 1993;329:611‐615. [DOI] [PubMed] [Google Scholar]
- 10. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69‐72. [DOI] [PubMed] [Google Scholar]
- 11. Wang L, Liu W, Yu Y, Jiang L, Yang J. Increased circulating bioactive c‐type natriuretic peptide is associated with reduced heart rate variability in patients with chronic kidney disease. BMC Nephrol. 2018;19:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reference Values for Arterial Stiffness Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. 2010;31:2338‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Consequences of orthostatic blood pressure variability in middle‐aged men (the malmo preventive project). J Hypertens. 2010;28:551‐559. [DOI] [PubMed] [Google Scholar]
- 15. Canney M, O'Connell M, Sexton DJ, et al. Graded association between kidney function and impaired orthostatic blood pressure stabilization in older adults. J Am Heart Assoc. 2017;6(5):e005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fedorowski A, Burri P, Melander O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J Hypertens. 2009;27:976‐982. [DOI] [PubMed] [Google Scholar]
- 17. Martin W, Smith JA, White DG. The mechanisms by which haemoglobin inhibits the relaxation of rabbit aorta induced by nitrovasodilators, nitric oxide, or bovine retractor penis inhibitory factor. Br J Pharmacol. 1986;89:563‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anand IS, Chandrashekhar Y, Wander GS, Chawla LS. Endothelium‐derived relaxing factor is important in mediating the high output state in chronic severe anemia. J Am Coll Cardiol. 1995;25:1402‐1407. [DOI] [PubMed] [Google Scholar]
- 19. Ni Z, Morcos S, Vaziri ND. Up‐regulation of renal and vascular nitric oxide synthase in iron‐deficiency anemia. Kidney Int. 1997;52:195‐201. [DOI] [PubMed] [Google Scholar]
- 20. Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epidemiology, prognosis, and treatment. J Am Coll Cardiol. 2015;66:848‐860. [DOI] [PubMed] [Google Scholar]
- 21. Pries AR, Neuhaus D, Gaehtgens P. Blood viscosity in tube flow: dependence on diameter and hematocrit. Am J Physiol. 1992;263:H1770‐H1778. [DOI] [PubMed] [Google Scholar]
- 22. Connes P, Martin C, Barthelemy JC, et al. Nocturnal autonomic nervous system activity impairment in sickle cell trait carriers. Clin Physiol Funct Imaging. 2006;26:87‐91. [DOI] [PubMed] [Google Scholar]
- 23. Martins Wde A, Lopes HF, Consolim‐Colombo FM, Gualandro Sde F, Arteaga‐Fernandez E, Mady C. Cardiovascular autonomic dysfunction in sickle cell anemia. Auton Neurosci. 2012;166:54‐59. [DOI] [PubMed] [Google Scholar]
- 24. Perera R, Isola L, Kaufmann H. Effect of recombinant erythropoietin on anemia and orthostatic hypotension in primary autonomic failure. Clin Auton Res. 1995;5:211‐213. [DOI] [PubMed] [Google Scholar]
- 25. Winkler AS, Landau S, Watkins PJ. Erythropoietin treatment of postural hypotension in anemic type 1 diabetic patients with autonomic neuropathy: a case study of four patients. Diabetes Care. 2001;24:1121‐1123. [DOI] [PubMed] [Google Scholar]
- 26. Kawakami K, Abe H, Harayama N, Nakashima Y. Successful treatment of severe orthostatic hypotension with erythropoietin. Pacing Clin Electrophysiol. 2003;26:105‐107. [DOI] [PubMed] [Google Scholar]
- 27. Ammarguellat F, Gogusev J, Drueke TB. Direct effect of erythropoietin on rat vascular smooth‐muscle cell via a putative erythropoietin receptor. Nephrol Dial Transplant. 1996;11:687‐692. [DOI] [PubMed] [Google Scholar]
- 28. Vaziri ND. Mechanism of erythropoietin‐induced hypertension. Am J Kidney Dis. 1999;33:821‐828. [DOI] [PubMed] [Google Scholar]
- 29. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long‐term adverse outcomes with early vs later orthostatic hypotension assessment times in middle‐aged adults. JAMA Intern Med. 2017;177:1316‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chou RH, Liu CJ, Chao TF, et al. Association between orthostatic hypotension, mortality, and cardiovascular disease in asians. Int J Cardiol. 2015;195:40‐44. [DOI] [PubMed] [Google Scholar]
- 31. Liu W, Wang L, Huang X, Yuan C, Li H, Yang J. Orthostatic blood pressure reduction as a possible explanation for memory deficits in dialysis patients. Hypertens Res. 2019;42(7):1049‐1056. [DOI] [PubMed] [Google Scholar]
- 32. Peters R, Anstey KJ, Booth A, et al. Orthostatic hypotension and symptomatic subclinical orthostatic hypotension increase risk of cognitive impairment: an integrated evidence review and analysis of a large older adult hypertensive cohort. Eur Heart J. 2018;39:3135‐3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McMurray J, Parfrey P, Adamson JW, et al. Kidney disease: improving global outcomes (kdigo) anemia work group. kdigo clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279. [Google Scholar]
- 34. Abramson JL, Jurkovitz CT, Vaccarino V, Weintraub WS, McClellan W. Chronic kidney disease, anemia, and incident stroke in a middle‐aged, community‐based population: the aric study. Kidney Int. 2003;64:610‐615. [DOI] [PubMed] [Google Scholar]
- 35. Fedorowski A, Wahlstrand B, Hedner T, Melander O. Systolic and diastolic component of orthostatic hypotension and cardiovascular events in hypertensive patients: the captopril prevention project. J Hypertens. 2014;32:75‐81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


