Abstract
Introduction
Exercise is recommended to improve glycaemic control. Yet, individual changes in glycaemic control following exercise can vary greatly, meaning while some significantly improve others, coined ‘non-responders’, do not. Increasing the intensity of exercise may ‘rescue’ non-responders and help generate a response to training. This trial will identify non-responders to changes in glycated haemoglobin (HbA1c) across inactive individuals living with pre-diabetes or type 2 diabetes mellitus following an aerobic exercise programme and evaluate if increasing training intensity will elicit beneficial changes to ‘rescue’ previously categorised non-responders.
Methods and analysis
This study will recruit 60 participants for a two-phase aerobic exercise training programme. Participants will be allocated to a control group or assigned to an intervention group. Control participants will maintain their current lifestyle habits. During phase 1, intervention participants will complete 16 weeks of aerobic exercise at an intensity of 4.5 metabolic equivalents (METs) for 150 min per week. Participants will then be categorised as responders or non-responders based on the change in HbA1c. For phase 2, participants will be blocked based on responder status and randomly allocated to a maintained intensity, or increased intensity group for 12 weeks. The maintained group will continue to train at 4.5 METs, while the increased intensity group will train at 6.0 METs for 150 min per week.
Ethics and dissemination
Results will be presented at scientific meetings and submitted to peer-reviewed journals. Publications and presentations related to the study will be authorised and reviewed by all investigators. Findings from this study will be used to provide support for future randomised control trials. All experimental procedures have been approved by the Research Ethics Board at the University of New Brunswick (REB: 2018–168).
Trial registration number
Keywords: diabetes & endocrinology, sports medicine, general diabetes
Strengths and limitations of this study.
Participants will be allocated, not randomised, to control and intervention groups for phase 1, as data from the control group is required to estimate variance and allow for the accurate categorisation of intervention participants prior to subsequent randomisation to exercise intensity branches in phase 2.
In line with recommendations from numerous governing bodies and policy-makers, the physical activity intervention will have participants complete 150 min of aerobic physical activity per week.
Multiple measurements of the primary outcome are taken at each time point to increase reliability.
An absolute measure of exercise intensity will be prescribed to equalise energy expenditure across all participants.
Introduction
Organisations around the globe provide standardised exercise recommendations to reduce the onset of chronic disease and premature mortality.1–5 However, observed changes following a typical exercise programme are often heterogeneous. This heterogeneity can result in individuals not experiencing the desired benefits of standard exercise training, and being labelled as ‘exercise non-responders’. Attempts to quantify the observed heterogeneity, known as interindividual variation, and/or estimate the prevalence of non-responders have recently proliferated.6–15
Research designed to identify non-responders has primarily focused on cardiorespiratory fitness in apparently healthy adults. Moreover, attempts to categorise youth6 15 and adult9 11 13 14 participants as responders or non-responders based on cardiometabolic outcomes have not often included a time-matched control group in their analysis. Opting to use single-group study designs or reliability data to set response thresholds and categorise participants, produces response rates which reflect the number of participants who improved beyond an estimate of random or measurement error10 16 17. Alternatively, including a time-matched control group allows an estimate of within-subject variation to be considered when setting a response threshold or calculating individual confidence intervals, encapsulating additional variance and accounting for its impact when making categorisations.10 18 19
Labelling individuals who do not experience the intended benefits following an exercise programme as non-responders can be problematic for several reasons, including the substantial influence a subjectively chosen threshold has on response categorisations, the high likelihood that a non-responder experienced a beneficial change in a secondary outcome, and the specificity of a response categorisation to the provided intervention.10 11 20 21 Accordingly, adapting exercise protocols for individuals initially categorised as non-responders may garner beneficial changes in the primary outcome, and thereby ‘rescue’, those individuals from their non-responder status.22 Montero and Lundby12 highlighted the potential effectiveness of such efforts, using a 120 min increase in weekly exercise volume to rescue a group of 21 apparently healthy, young adult, male non-responders from their original categorisation.12 Less is known, however, about the ability of adapting exercise training intensity to rescue non-responders. One study from Ross et al8 allocated 121 sedentary adults living with obesity to one of three groups for 24 weeks of exercise training: (1) low volume, low intensity; (2) high volume, low intensity or (3) high volume, high intensity.8 The authors found doubling the training volume (group 1 vs 2) led to a 50% decrease in non-response, whereas increasing the intensity and volume (group 3) only produced responders. While these findings suggest an increase in exercise intensity can increase the overall proportion of responders, it remains unknown if this would translate to specific improvements in those previously categorised as non-responders. Moreover, no such work has been conducted with those living with pre-diabetes or type 2 diabetes mellitus (T2DM) or using an indicator of glycaemic control as the primary outcome.
The INTENSITY study is a two-phase, quasi-experimental trial. The objectives are to:
Identify the number of exercise non-responders, based on the observed changes in glycated haemoglobin (HbA1c), across individuals living with pre-diabetes or T2DM following 16 weeks of continuous aerobic exercise training.
Explore if increasing the intensity and/or increasing the duration of exercise training by 12 weeks will ‘rescue’ previously identified non-responders by garnering improvements in HbA1c.
For the purpose of this analysis, an exercise responder will be defined as any individual who has experienced a decrease in HbA1c beyond the minimal clinically important difference (MCID) following participation in the provided exercise trail, while accounting for the variation-induced changes in HbA1c experienced by the time-matched control group. We hypothesise that a significant proportion of participants will be categorised as non-responders following participation in the exercise programme, and increasing the intensity of exercise training will rescue the previously identified non-responders by producing beneficial changes in HbA1c.
Methods and analysis
Study setting
The INTENSITY trial will be conducted at the University of New Brunswick in Fredericton, New Brunswick, Canada. This location was chosen due to the available equipment, ease of access for participants, availability of a private exercise facility for the delivery of the training protocol, and the relatively high rates of T2DM throughout the province.23
Eligibility criteria
Inclusion criteria
Community-dwelling adults aged 19 years or older.
Currently living with pre-diabetes or T2DM as diagnosed by a physician and confirmed by an HbA1c value of 5.7% or above, as verified by duplicate testing.
Not currently partaking in a self-reported regular physical activity regimen, defined as consistent participation in running or jogging activity, attending physical activity or exercise classes on a weekly basis, or averaging 10 000 steps per day or more over the course of 7 days.
Exclusion criteria
Self-reported diagnosis of low iron concentrations, anaemia, or being treated for these conditions.
Diagnosed with any red blood cell altering condition.
Currently living with any cardiovascular disease which would impact the ability to safely participate in exercise training.
Currently prescribed any medication which would impact the ability to use a heart rate monitor to accurately track exercise intensity.
Recruitment
Participants will be recruited from the city of Fredericton, New Brunswick, Canada, and the surrounding area using advertisements placed in participating grocery stores, pharmacies, healthcare centres, physician offices and on social media. The research team will also use internal newsletters and electronic communication platforms to inform staff and students at the University of New Brunswick and St. Thomas University of the study. Partnerships with the local branches of Diabetes Canada and government-funded diabetes education and support programmes will allow for research staff to attend meetings and distribute advertisements to clients.
Patient and public involvement
Prior to designing the study, 65 patients living with T2DM in Fredericton, New Brunswick and the surrounding area who previously engaged in an exercise-based lifestyle intervention programme were consulted by the research team to help ensure relevance of the research purpose to this population, and provide effective dissemination input. As a results, findings will be provided to study participants on an individual basis via their requested means of communication, and the research team will host a public event to discuss the findings, what they mean, and how they may be implemented by interested stakeholders.
Interventions
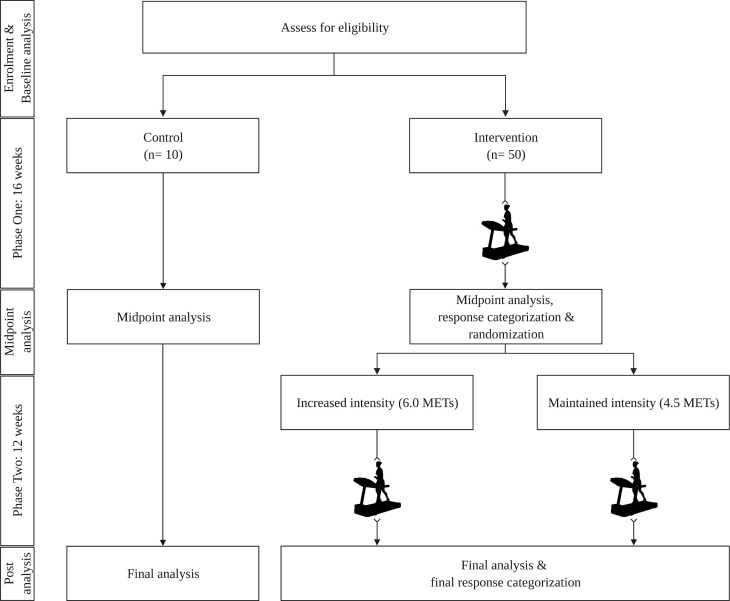
Participation in the INTENSITY trial will take place across two distinct phases (figure 1).
Figure 1.
Participant flow chart. METs, metabolic equivalents. Created with BioRender.com.
Phase 1
Eligible participants will be assigned to one of a control or intervention group. Allocation will be based on the time of recruitment, with the first participants assigned to the control condition until group capacity is reached. All subsequent participants will be assigned to the intervention. Allocation was chosen in favour of randomisation during phase 1, as data from the control group is required to estimate measurement variance and allow for the accurate categorisation of intervention participants prior to randomisation in phase 2.
Participants allocated to the control group will receive no exercise advice or instruction. Control participants will be instructed to maintain current lifestyle habits, contacted monthly to ensure continued enrollment and answer pertinent questions, and asked to return for midpoint testing in 16 weeks. Participants allocated to the intervention group will be scheduled to begin the first phase of the training protocol within 1 week of completing all baseline testing. The phase 1 training protocol will last for 16 weeks, requiring participants to exercise at an intensity of 4.5 metabolic equivalents (METs) on a treadmill. An absolute measure of intensity was chosen in favour of a relative measure of intensity to equalise energy expenditure across all participants. All exercise will be supervised by research staff and take place in a private exercise facility located on the University of New Brunswick campus. To maximise attendance exercise sessions will be scheduled on a weekly basis. Participants will be eased into the programme using a 4-week progression, completing 80 min of exercise in week 1, 100 min in week 2, 120 min in week 3 and 135 min in week 4. For each of the remaining 12 weeks, participants will complete 150 min of exercise. Each participant will choose the number of weekly sessions needed to complete the required time, as long as the total number of sessions is greater than one. Participants will be allowed to choose the speed and grade of the treadmill during the exercise time, as long as the prescribed intensity is achieved and maintained for the duration of each session. The supervising research staff will instruct participants to increase or decrease exercise intensity by increasing or decreasing the speed and/or grade of the treadmill, as necessary. At the start of every training session participants will be given 5 min to warmup and achieve the targeted intensity. Warmup time during each session will not count towards the total exercise time for the week. To account for improvements in cardiorespiratory fitness and ensure participants train at the appropriate intensity, each participant will be re-evaluated every 4 weeks.
Categorisation of exercise responders and non-responders
Based on the observed changes in HbA1c following the 16 weeks of exercise training, each participant in the intervention group will be categorised as a responder, non-responder or uncertain responder. Individual 90% CI around the observed change in HbA1c will be calculated for each participant, with participants whose lower bound of the 90% CI lays above the selected response threshold categorised as responders, and those whose upper bound of the 90% CI lays below the response threshold categorised as non-responders. As the CI for some participants may cross the threshold for response, it may not be possible to confidently categorise all participants as either a responder or non-responder. Therefore, those participants whose 90% CI partially crosses the response threshold will be categorised as uncertain responders. For the purpose of randomisation in phase 2, participants categorised as uncertain will be grouped with non-responders.
Phase 2
Participants in the control group will again be instructed to maintain their current lifestyle habits, contacted monthly to answer any pertinent questions, and asked to return for follow-up testing in 12 weeks. Participants in the intervention group will be blocked based on their responder status and randomly allocated to either a maintained exercise group, or an increased intensity group. Participants in the maintained exercise group will continue the supervised, treadmill-based aerobic exercise training for 150 min per week at an intensity of 4.5 METs, for 12 weeks. Participants in the increased intensity group will increase the intensity of their supervised, treadmill-based aerobic exercise to 6.0 METs, for 150 min per week. Participant scheduling, freedom to choose treadmill speed and slope, and supervision will follow the same methods as applied during Phase One. Likewise, cardiorespiratory fitness will be re-evaluated every 4 weeks.
Deviations from protocol
Research staff will emphasise that each participant receives the same dose of exercise (time and energy expenditure), as differences throughout the intervention group can have negative repercussions on the SDIR.24 Accordingly, enrolment will be discontinued if a participant is unable to achieve the required time allotment for three consecutive weeks, or for a total of 4 weeks during either phase 1 or phase 2. If a participant is absent from the trial for a full week (due to illness, vacation, family emergency, etc), an additional week will be added at the end of the trial for that participant for each week missed. A maximum of 3 weeks throughout the totality of the trial may be added for a single participant, at which point the participant will be excluded from further participation. The reason provided for missing a week of training will be documented and available for interpretation when conducting the final analysis. Enrolment will also be discontinued if a participant experiences any musculoskeletal injury or other medical event which prevents or limits safe participation in exercise for three consecutive weeks, or if the participant receives medical advice to stop participation. Data from these participants will be kept to allow for baseline comparisons. Likewise, if the injury or medical event takes place during phase Two2, the participant’s phase 1 data will be included in the analysis, and the reason for exclusion clearly indicated.
Data collection and management
Participant files will be deidentified, and each participant will be assigned a unique identifier at the time of first contact with the research staff. All participants will meet with the research staff for the sole purpose of data collection six times across three timepoints: twice at the time of enrolment for baseline evaluation, twice between phase 1 and phase 2 for midpoint follow-up and to allow for randomisation, and twice following phase 2 for post-testing (table 1). Additionally, participants’ heart rate, chosen treadmill speed, and chosen treadmill slope will be recorded in 5 min segments throughout the duration of every exercise training session. All data will be collected in written form, and subsequently transferred to electronic files. Physical versions of all files will be stored locally, in a secure room at the University of New Brunswick. Digital files will be housed on a secure server operated by the University of New Brunswick.
Table 1.
Measurement timeline
| Weeks | 1 | 2–17 | 18 | 19–31 | 32 | ||||
| Visit | 1 | 2 | Phase 1 | 3 | 4 | Phase 2 | 5 | 6 | |
| Demographics and family history | X |
16 weeks exercise training (Approximately 3–5 visits per week) Submaximal fitness re-evaluation every 4 weeks |
Randomisation |
12 weeks exercise training (Approximately 3–5 visits per week) Submaximal fitness re-evaluation every 4 weeks |
|||||
| Medication use | X | X | |||||||
| Anthropometrics and blood pressure | X | X | X | ||||||
| Glycaemic control | X | X | X | X | X | X | |||
| Cardiorespiratory fitness | X | X | X | ||||||
| Physical Activity & Sedentary Behaviour | X | X | |||||||
Outcomes and instrumentation
Primary outcome
The primary outcome of the INTENSITY trail is HbA1c, analysed using a DCA Vantage Analyzer (Siemens, Germany). A finger prick will be conduced using a Safe-T Pro Plus single use lancet (Accu-Chek, Roche Diagnostics, Switzerland) to allow for the collection of 1 μL of whole blood. The sample will then be loaded into the DCA Vantage Analyzer, where a rapid assessment of HbA1c is conducted, providing results in approximately 6 min. The DCA Vantage Analyzer has been shown to provide accurate, valid measures of HbA1c when compared with laboratory measurements.25–27 To further increase reliability HbA1c will be measured twice at each timepoint, separated by less than 7 days, with the mean value used in all analyses. The potential influence of measurement error will be estimated by calculating the typical error (see below).
Secondary outcomes
At baseline research staff will record participant demographics, family history of cardiovascular and cardiometabolic disease, and current medication use. Participants will be monitored by the research staff throughout the study and required to report any changes in medication use. These changes will be confirmed at each testing time point. To confirm current physical activity patterns and ensure eligibility, participants will then complete the Physical Activity and Sedentary Behaviour Questionnaire to assess current physical activity and sedentary behaviours,28 and will be sent home with a Piezo Rx pedometer (StepsCount, Deep River, Ontario, Canada). Research staff will instruct each participant to wear the pedometer for seven consecutive days, excluding sleep time, and to remove the pedometer prior to any activity with the potential to submerge the device in water. If the pedometer is lost or not worn, participants will be required to wear the device for another 7 days.
At each timepoint physiological and anthropometric measurements will occur over the span of 2 days, separated by less than 1 week. Participant height, systolic and diastolic blood pressure, and waist circumference will be measured by a member of the research team in accordance with Canadian Society for Exercise Physiology protocols.28 Body mass, fat mass and fat free mass will be estimated using the BODPOD (COSMED; Rome, Italy) following a 12-hour overnight fast. The BODPOD is a highly valid and reliable method for assessing body composition.29 30 All cardiorespiratory fitness [Volume of oxygen (VO2peak)] evaluations will be supervised by TH. The original Balke and Ware treadmill test protocol31 has been amended for this study, to fit within the restrictions of the available equipment. Participants will walk at 3.4 miles per hour (mph) at 0% grade on a treadmill (9500HR (Life Fitness, Illinois, USA)). After 2 min, the grade will be increased to 5.0%, and progressively increase by 1.0% every minute thereafter until 15.0% is achieved. If the participant is not fatigued, the grade will be maintained, and the speed increased by 0.5 mph each minute until volitional fatigue. Gas exchange and heart rate will be continuously gathered using a TrueOne 2400 Metabolic Cart (ParvoMedics, Salt Lake City, Utah, USA) and Polar FT1 heart rate monitor (Polar, Kempele, Finland), respectively. VO2peak will be identified as the highest achieved 15 s average VO2. Following the treadmill test, participant METs and heart rate values will be reviewed by TH, and the heart rate associated with an intensity of 4.5 or 6.0 METs (in line with the current exercise prescription) will be identified. The identified heart rate value will be used to prescribe and monitor participant intensity during subsequent training sessions, until cardiorespiratory fitness is re-evaluated. Should the exact MET value not be observed during the test, the next closest value below the desired MET value (ie, 4.4 or 5.9 METS) will be used. The same research staff member (TH) will be present at each assessment to reduce the potential for inter-rater differences to skew results.
Cardiorespiratory fitness re-evaluation
Every 4 weeks throughout each phase, a staff member will re-evaluate each participant to ensure adaptations in cardiorespiratory fitness are accounted for. Replicating a typical exercise session, participants will warm-up and workout while gas exchange and heart rate are recorded using a TrueOne 2400 Metabolic Cart (Parvomedics) and Polar FT1 heart rate monitor (Polar, Kempele, Finland), respectively. The participant will remain in control of the treadmill grade and speed and instructed to increase intensity as needed until the desired MET value (4.5 or 6.0 METs) is achieved and maintained for a period of 1 min, as observed by the supervising staff member. Exercise accumulated during the re-evaluation will count towards the required weekly training time. The target heart rate identified during the fitness re-evaluation will be used for all subsequent training sessions, until the next re-evaluation is completed. The same research staff member (TH) will review the results of every re-evaluation to ensure consistency throughout the trial. A submaximal re-evaluation protocol was chosen in place of repeated administration of the maximal testing protocol to avoid the potential influence of repeated maximal tests on the outcomes, and to maximise participant comfort and compliance.
Exercise monitoring
To ensure participants are exercising at the appropriate intensity, research staff will monitor and record participant heart rate in 5 min intervals using the Polar Team2 (Polar, Kempele, Finland) heart rate monitoring system, treadmill speed and treadmill slope. The supervising research staff member will ensure each participant’s heart rate throughout each training session remains within ±2.5% of heart rate associated with the assigned MET value (as identified during the cardiorespiratory fitness test or subsequent re-evaluation).
Blinding
Participants and all research staff who assess, train or otherwise interact with participants will be blinded to all follow-up measures of HbA1c, as well as the participant responder categorisation. To maintain blinding, research staff who do not normally interact with participants will be responsible for the collection and recording of HbA1c results, determining individual participant responder status, and completing the randomisation process. Group allocation for phase 2 will then be told to TH, who will disseminate the required training protocol to other research staff and participants.
Randomisation
Randomisation of intervention participants will occur after the follow-up assessment prior to phase 2. A member of the Cardiometabolic Exercise and Lifestyle Laboratory (CELLAB) staff not related to the project will collect the HbA1c values following phase 1, calculate the participant’s change score, and determine the response categorisation, which will be communicated via email to DRB, who has no contact with participants. The response categorisation will be entered into a random number generator (SPSS V.22.0) to decide the phase 2 arm allocation. Randomisation will occur in blocks of 10 (five per group) based on participant response status (responder vs non-responder and uncertain responder). Blocks of five for each group were chosen for the randomisation procedure due to the inability to predict the proportion of participants which would be categorised as responders or non-responders/uncertain responders, as a method to reduce potential biases and maximise the likelihood of achieving balance between the maintained intensity and increased intensity groups. The phase 2 arm for the participant will be communicated to TH via email by the member of the CELLAB staff.
Statistical analysis
The sample size calculation was based on the anticipated change in the primary outcome measure, HbA1c, following phase 1 of the trial. A meta-analysis conducted by Umpierre et al,32 indicates supervised aerobic exercise training of 12 weeks or longer is associated with a 0.73% reduction in HbA1c, which is anticipated here. Given a desired power of 80% and alpha of 0.05, a generalised linear model with a linear auto-regression structure (to account for the duplicate measures of HbA1c) was used to calculate the necessary sample size. A sample of 42 participants was identified to detect significant changes in HbA1c in phase 1 of the trial. Provided an anticipated 20% drop-out rate, 50 participants will be recruited to participate in the intervention group. All participants who complete phase 1 are anticipated to continue and complete phase 2. An additional 10 participants will be recruited for allocation to the time-matched control group.
Objective 1: Identify the number of exercise non-responders, based on the observed changes in HbA1c, across individuals living with pre-diabetes or T2DM following 16 weeks of continuous aerobic exercise training. Individuals will be categorised as responders if the observed change can confidently be assumed to be beyond the MCID, while accounting for the variation-induced changes experienced by the time-matched control group. Accordingly, individual 90% CI for each participant will be calculated using the equation outlined by Swinton et al.19:
Individual CI = (Observed scoreMID – Observed scoreBASELINE) ± (CI multiplier x SDCON).
Here, the SDCON allows for potential variation introduced in the absence of the intervention to be considered when constructing each CI. As the sample size of the control group may influence the certainty of the CI, the CI multiplier will be adjusted for a control sample size of 10 individuals (CI multiplier=1.83).19 The individual CIs will be evaluated against a threshold of clinical relevance. In line with the Federal Drug Agency and the European Medicines Agency, an MCID of 0.3% for HbA1c will be used.33 34
Objective 2: Explore if increasing the intensity and/or increasing the duration of exercise training will ‘rescue’ previously identified non-responders by garnering improvements in HbA1c. Following the completion of phase 2, the categorisation procedure conducted during objective 2 will be repeated for all previously identified uncertain and non-responders, with important adjustments made to the CI equation. The SDCON will be recalculated with the SD of the pre–post difference scores from the control group using HbA1c measurements taken at midpoint and follow-up post testing. This will allow for the CIs to represent the variation-induced changes experienced by the time-matched control group across the second, 12-week period for objective 2.
Accordingly, the individual 90% CIs will be calculated using the equation outlined by Swinton et al.19 :
Individual CI = (Observed scorePOST – Observed scoreMID) ± (CI multiplier x (SDCON)).
Individual CIs will again be evaluated against the MCID response threshold of 0.3%, and participants recategorised as previously described. The raw number of participants who were previously categorised as a non-responder or uncertain responder that are categorised as responders after completing phase 2 will be reported.
Ethics and dissemination
All experimental procedures have been approved by the Research Ethics Board at the University of New Brunswick (REB: 2018–168). Any substantial protocol amendments will be sent to the Research Ethics Board for review and approval prior to implementation.
Informed consent
At the time of first contact with a member of the research staff, interested individuals will be provided with information about the study and have their eligibility confirmed. Eligible individuals will be provided with a digital copy of the consent form prior to the first meeting. At the initial meeting, all eligible individuals will be provided with adequate time to review a physical copy of the consent form (online supplemental file 1), ask any questions, and consider their participation. If the individual decides to become a participant, they will be asked to provide written consent, which will be countersigned by the research staff. All participants are free to withdraw from the study at any time.
bmjopen-2020-044478supp001.pdf (7.7MB, pdf)
Dissemination
Results will be presented at scientific meetings and submitted to peer-reviewed journals. All publications and presentations related to the study will be authorised and reviewed by all investigators. Findings from this study will be used to develop and provide support for future randomised control trials. All study participants will have the option at the time of consent to request a copy of the study findings at the time of completion. A summary of the findings will be provided to all participants who indicate a desire to receive it.
Trial status
The study is currently recruiting and enrolling participants. The first participant was recruited in May 2019, and recruitment is expected to be complete in November 2020. The expected completion date of the project, including all follow-up appointments, is June 2021.
Supplementary Material
Footnotes
Contributors: MS is the primary investigator. DB, BG and TH contributed to the study design and analysis methods. TH will coordinate study implementation and data collection. All authors contributed to the completion and approved the final version of this manuscript. The authors would also like to thank the patient advisers who contributed to the study.
Funding: This work is supported by grants from The Heart and Stroke Foundation New Brunswick (unspecified award number) and New Brunswick Health Research Foundation (2018–2019). TH salary was supported by a combined New Brunswick Health Research Foundation and Maritime SPOR Support Unit studentship (2017-MSSUSF-1246).
Disclaimer: The study sponsor had no input to the design, implementation, interpretation, or any decision making of this study.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Tremblay MS, Warburton DER, Janssen I, et al. New Canadian physical activity guidelines. Appl Physiol Nutr Metab 2011;36:36–46. 10.1139/H11-009 [DOI] [PubMed] [Google Scholar]
- 2.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018;320:2020–8. 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diabetes Canada clinical practice guidelines expert Committee . Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2018:S1–325. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Global recommendations on physical activity for health. WHO Press, 2010. [PubMed] [Google Scholar]
- 5.Brown WJ, Bauman AE, Bull F. Development of evidence-based physical activity recommendations for adults (18-64 years). Australia: Commonwealth of Australia, 2013. [Google Scholar]
- 6.Alvarez C, Ramírez-Campillo R, Ramírez-Vélez R, et al. Effects of 6-Weeks high-intensity interval training in schoolchildren with insulin resistance: influence of biological maturation on metabolic, body composition, cardiovascular and performance Non-responses. Front Physiol 2017;8:444. 10.3389/fphys.2017.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurd BJ, Giles MD, Bonafiglia JT, et al. Incidence of nonresponse and individual patterns of response following sprint interval training. Appl Physiol Nutr Metab 2016;41:229–34. 10.1139/apnm-2015-0449 [DOI] [PubMed] [Google Scholar]
- 8.Ross R, de Lannoy L, Stotz PJ. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin Proc 2015;90:1506–14. 10.1016/j.mayocp.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 9.Bouchard C, Blair SN, Church TS, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One 2012;7:e37887. 10.1371/journal.pone.0037887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hecksteden A, Pitsch W, Rosenberger F, et al. Repeated testing for the assessment of individual response to exercise training. J Appl Physiol 2018;124:1567–79. 10.1152/japplphysiol.00896.2017 [DOI] [PubMed] [Google Scholar]
- 11.Phillips BE, Kelly BM, Lilja M, et al. A practical and Time-Efficient high-intensity interval training program modifies Cardio-Metabolic risk factors in adults with risk factors for type II diabetes. Front Endocrinol 2017;8:229. 10.3389/fendo.2017.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montero D, Lundby C. Refuting the myth of non-response to exercise training: 'non-responders' do respond to higher dose of training. J Physiol 2017;595:3377–87. 10.1113/JP273480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lannoy L, Clarke J, Stotz PJ, et al. Effects of intensity and amount of exercise on measures of insulin and glucose: analysis of inter-individual variability. PLoS One 2017;12:e0177095. 10.1371/journal.pone.0177095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leifer ES, Mikus CR, Karavirta L, et al. Adverse cardiovascular response to aerobic exercise training: is this a concern? Med Sci Sports Exerc 2016;48:20–5. 10.1249/MSS.0000000000000752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sénéchal M, Rempel M, Duhamel TA, et al. Fitness is a determinant of the metabolic response to endurance training in adolescents at risk of type 2 diabetes mellitus. Obesity 2015;23:823–32. 10.1002/oby.21032 [DOI] [PubMed] [Google Scholar]
- 16.Hecksteden A, Kraushaar J, Scharhag-Rosenberger F, et al. Individual response to exercise training - a statistical perspective. J Appl Physiol 2015;118:1450–9. 10.1152/japplphysiol.00714.2014 [DOI] [PubMed] [Google Scholar]
- 17.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med 2000;30:1–15. 10.2165/00007256-200030010-00001 [DOI] [PubMed] [Google Scholar]
- 18.Hopkins WG. Individual responses made easy. J Appl Physiol 2015;118:1444–6. 10.1152/japplphysiol.00098.2015 [DOI] [PubMed] [Google Scholar]
- 19.Swinton PA, Hemingway BS, Saunders B, et al. A statistical framework to interpret individual response to intervention: paving the way for personalized nutrition and exercise prescription. Front Nutr 2018;5:41. 10.3389/fnut.2018.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross R, Goodpaster BH, Koch LG, et al. Precision exercise medicine: understanding exercise response variability. Br J Sports Med 2019;53:1141–53. 10.1136/bjsports-2018-100328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh JJ, Bonafiglia JT, Goldfield GS, et al. Interindividual variability and individual responses to exercise training in adolescents with obesity. Appl Physiol Nutr Metab 2020;45:45–54. 10.1139/apnm-2019-0088 [DOI] [PubMed] [Google Scholar]
- 22.Marsh CE, Thomas HJ, Naylor LH, et al. Fitness and strength responses to distinct exercise modes in twins: studies of twin responses to understand exercise as a therapy (STRUETH) study. J Physiol 2020;598:3845–58. n/a. 10.1113/JP280048 [DOI] [PubMed] [Google Scholar]
- 23.Gupta N. Charting the progression of diabetes mellitus in new Brunswick: rates, correlates, and implications for accountability in public policy. 1 published online first: 23 November 2017. Available: https://journals.lib.unb.ca/index.php/JNBS/article/view/25880 [Accessed 25 Jul 2019].
- 24.Bonafiglia JT, Brennan AM, Ross R, et al. An appraisal of the SDIR as an estimate of true individual differences in training responsiveness in parallel-arm exercise randomized controlled trials. Physiol Rep 2019;7:e14163. 10.14814/phy2.14163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez-Mora C, S Rodríguez-Oliva M, Fernández-Riejos P, et al. Evaluation of two HbA1c point-of-care analyzers. Clin Chem Lab Med 2011;49:653–7. 10.1515/CCLM.2011.101 [DOI] [PubMed] [Google Scholar]
- 26.Mardis C, Foohey L. Laboratory-Quality hemoglobin A1c results at the point of care with DCA Vantage analyzer. Point Care 2017;16:63–6. 10.1097/POC.0000000000000130 [DOI] [Google Scholar]
- 27.Szymezak J, Leroy N, Lavalard E, et al. Evaluation of the DCA Vantage analyzer for HbA1c assay. Clinical Chemistry and Laboratory Medicine 2008;46:1195–8. 10.1515/CCLM.2008.228 [DOI] [PubMed] [Google Scholar]
- 28.Canadian Society for Exercise Physiology . Canadian Society for Exercise Physiology - Physical Activity Training for Health (CSEP-PATH). Canadian Society for Exercise Physiology 2013. [Google Scholar]
- 29.Bailey BW, Tucker LA, Peterson TR, et al. Test-Retest reliability of body fat percentage results using dual energy X-ray absorptiometry and the BOD pod. Medicine & Science in Sports & Exercise 2001;33:S174. 10.1097/00005768-200105001-00988 [DOI] [Google Scholar]
- 30.Vescovi JD, Zimmerman SL, Miller WC, et al. Evaluation of the BOD pod for estimating percentage body fat in a heterogeneous group of adult humans. Eur J Appl Physiol 2001;85:326–32. 10.1007/s004210100459 [DOI] [PubMed] [Google Scholar]
- 31.Balke B, Ware RW. The present status of physical fitness in the air force. Proj Rep USAF Sch Aviat Med 1959;59:675–88. [PubMed] [Google Scholar]
- 32.Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–9. 10.1001/jama.2011.576 [DOI] [PubMed] [Google Scholar]
- 33.Center for Drug Evaluation and Research . Guidance for industry diabetes mellitus: devleoping drugs and therapeutic biologics for treatment and prevention, 2008. [Google Scholar]
- 34.Committee for Medicinal Products for Human Use . Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus, 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-044478supp001.pdf (7.7MB, pdf)