Abstract
Food frequency questionnaires (FFQs) are often used to assess dietary sodium intake, although 24‐hour urinary excretion is the most accurate measure of intake. The authors conducted a systematic review to investigate whether FFQs are a reliable and valid way of measuring usual dietary sodium intake. Results from 18 studies are described in this review, including 16 validation studies. The methods of study design and analysis varied widely with respect to FFQ instrument, number of 24‐hour urine collections collected per participant, methods used to assess completeness of urine collections, and statistical analysis. Overall, there was poor agreement between estimates from FFQ and 24‐hour urine. The authors suggest a framework for validation and reporting based on a consensus statement (2004), and recommend that all FFQs used to estimate dietary sodium intake undergo validation against multiple 24‐hour urine collections.
Keywords: diet surveys, sodium dietary, urine specimen collection
1. INTRODUCTION
Major international and national health and scientific organizations, as well as technical experts, have expressed strong concerns that low‐quality research on dietary salt is a major source of scientific controversy by causing artefactual “J‐shaped” or inverse relationships between dietary salt and cardiovascular disease.1, 2, 3, 4, 5 As a result, a major international consortium of health and scientific organizations was formed to set minimum standards for the conduct of clinical and epidemiological research on dietary salt.6 Accurate assessment of an individual's usual sodium intake is essential for investigation of relationships between dietary sodium intake and health outcomes in epidemiological studies, as well as for assessment of adherence in intervention studies.
There are considerable challenges in accurate measurement of usual sodium intake in individuals, including day‐to‐day variability in sodium intake.7 This means that several days of measurement are required to accurately measure usual intake with estimates of 3 to 10 days reported in the literature.8 Further challenges are specific to methods of assessment.
Dietary assessment tools and urinary sodium excretion are used to assess intake, with 24‐hour urinary excretion widely regarded as the most accurate measure of intake over a 24‐hour period. Food frequency questionnaires are often used in epidemiological studies as they assess dietary intake over a longer period, have a relatively low respondent burden, and can produce information about several nutrients, foods, and dietary patterns.9 Typically, food frequency questionnaires (FFQs) ask participants to record how frequently they have consumed particular foods over a specified time period (weeks or months) from a list of foods appropriate for the population under investigation. The food list should include foods consumed reasonably frequently by a substantial proportion of individuals in the population and contribute substantially to population intake of nutrient(s) of interest. The results of the questionnaire are analyzed with reference to a relevant food composition database to generate either estimates of intake or to rank intakes in individuals from high to low. Quantiles of intake are sometimes produced from FFQ survey data.10 There has been some dispute about the validity of the FFQ in epidemiologic studies,11, 12, 13 and its ability to characterize intake may vary by nutrient.
The degree to which FFQs can accurately measure sodium intake is not well understood, despite their use in many published studies. In a meta‐analysis of prospective studies examining salt intake and cardiovascular outcomes (including stroke and cardiovascular disease mortality) four of the 13 included studies used FFQs in their assessment of dietary sodium intake.14 Validation studies of FFQs are important to assess the degree to which they can accurately assess intakes of nutrients of interest. In such studies, FFQs are usually compared with other more reliable assessment methods such as other forms of dietary assessment or relevant biomarkers.9
Twenty‐four‐hour urinary sodium (a recovery biomarker) is widely regarded as the gold standard method for measurement of sodium intake. Approximately 85% to 90% of sodium ingested over a 24‐hour period is excreted in the urine, with the remainder excreted in sweat and feces. Twenty‐four‐hour urine collection places considerable burden on participants, and both under‐collection and over‐collection have been reported. Collection of 24‐hour urine over several days is likely to be the best method for assessment of usual sodium intake; however, in large population‐based epidemiological studies, the respondent burden associated with repeated 24‐hour urine may lead to low response rates.15 Nevertheless, 24‐hour urine is the most suitable reference method or calibration instrument for comparison in validation studies of dietary assessment methods,16, 17 and multiple collections over several days are most appropriate for validation of FFQs.
This article, commissioned by the TRUE (International Consortium for Quality Research on Dietary Sodium/Salt) consortium.11The TRUE consortium has a mandate to develop minimum standards for clinical and epidemiological research on dietary salt. Member organizations of the TRUE consortium include the American Heart Association, the British and Irish Hypertension Society, the Chinese Regional Office of the World Hypertension League, Hypertension Canada, the International Association of National Public Health Institutes, the International Council of Cardiovascular Prevention and Rehabilitation, the International Society of Hypertension, the International Society of Nephrology, the Journal of Clinical Hypertension, the World Health Organization Collaborating Centre for Population Salt Reduction, the Technical Advisory Group to mobilize cardiovascular disease prevention through dietary salt control policies and interventions, the Pan American Health Organization/World Health Organization, the World Hypertension League, and the World Stroke Organization. describes a systematic review of studies examining sodium intake assessment from FFQs compared with the gold standard 24‐hour urine collection, in order to understand whether FFQs are a reliable and valid way of measuring usual dietary sodium intake.
2. METHODS
2.1. Search strategy
The electronic databases MEDLINE, Embase, Cinharl, LILACS, Google Scholar, and the Cochrane Library were searched using suitable predefined terms in November 2015 (see Appendix A). An additional search was conducted in November 2016 to identify articles published during the interim period. Two authors (R.M. and V.F.) independently reviewed the titles and abstracts of all articles identified, discussed any disagreements, and achieved consensus. Potentially eligible articles were obtained in full text. Titles, abstracts, and full‐text articles published in languages other than English were translated into English. Both authors then independently reviewed the full‐text articles.
Reference lists of included studies were hand searched for additional articles not identified in the database search, and enquiries were made with coauthors and academic colleagues to identify further potentially eligible studies.
2.2. Eligibility criteria
Studies were eligible for inclusion if they were available in full text and undertaken in adult humans in free‐living settings. Feeding studies or studies where the amount of sodium in the diet was controlled by investigators were excluded. There were no restrictions on language or study sample size. Studies conducted within a population in an active disease state that may interfere with normal sodium metabolism, renal function, and urinary excretion (eg, renal failure, congestive heart failure, or pregnancy) were excluded. Studies were included if they reported dietary assessment of sodium intake (24‐hour diet recall, weighed diet record, and FFQs) and 24‐hour urinary collection for assessment of sodium intake in the same participants. Studies that collected urine samples over periods shorter than 24 hours were excluded. Only studies that reported on FFQs and 24‐hour urine collection are included in this analysis.
2.3. Data extraction
Standard data were extracted to a spreadsheet by two authors independently (R.M. and V.F.) and checked by a third author (A.N.) for accuracy. The variables recorded were study citation, study name, type of study (validation, cohort, or cross‐sectional), population studied (country, type of sample), participant characteristics (age, ethnicity, sex, disease status), whether and how 24‐hour urine collections were validated for completeness, 24‐hour urine results, dietary assessment methods and whether discretionary salt (defined as salt added either during cooking or at the table or both) was accounted for, dietary assessment results, whether dietary assessment and 24‐hour urine collections were concurrent, and what the methods of comparison were (if any) between the two methods.
Data were extracted for 18 studies that reported on results of FFQs and 24‐hour urinary sodium in the same participants (Table 1). Where data from more than one study were included in a single article,18 data from individual studies were extracted separately where possible. Where data from a single study were reported in two articles,19, 20 this was treated as one study. Supporting articles that described methods of data collection for studies were reviewed for additional data (particularly on methods) where required.
Table 1.
Characteristics of included studies (summary table)
| First author, year/name of study | Patients, No. | 24‐h Urine | Dietary assessment (FFQ) | Discretionary salt accounted for | 24‐h Urine and FFQ concurrent | Method(s) of comparison | |
|---|---|---|---|---|---|---|---|
| Bedford, 201121 | 102 | Single collection | FFQ (National Cancer Institute) (124 items), past 12 mo, analyzed with a Canadian version of the nutrient database | No | No | Correlation (0.21) | |
| Charlton, 200822 | 284 | Three collections, PABA, urine volume, and urinary creatinine concentration used to assess completeness | Salt‐specific FFQ (42 items), previous 7 d | Yes | Yes | Correlation (0.173); κ (0.0318) categorizing high (>2400 mg/d) vs low (<2400 mg/d) sodium intake | |
| Day, 2001/EPIC‐Norfolk cohort study23 | 123 | Six collections, PABA used to assess completeness | Two FFQs (130 items), 18 mo apartFFQ self‐administered, past 12 moModified questionnaire from US Nurses’ Health StudyCalculation based on Ministry of Agriculture, Fisheries, and Food Composition Tables | N/S | Yes | Sample variance, correlation (0.36), regression analysis | |
| Ferreira‐Sae, 200924 | 132 | Single collection | Salt‐specific FFQ (44 items), past 12 moNutwin database software developed by Federal University of Sao Paulo | In a separate questionnaire | No | Correlation | |
| Freedman, 2015/Nutrition Biomarker Study for WHI 2004–200518, 25 | 544 | Single collection, PABA and self‐report used to assess completenessTotal excretion divided by 0.86a | WHI FFQ, past 3 mo, nutrient databaseNutrition Data System for Research, University of Minnesota to analyze the results | N/S | No | Bias, attenuation factor of 0.12 (95% CI, 0.03–0.20), correlation adjusted for within‐person biomarker variation for pooled data | Pooled data from five studies, average correlation 0.16 (men 0.17, women 0.15), average attenuation factor of 0.08 (95% CI, 0.04–0.13) |
| Freedman, 2015/OPEN study 1999–200018, 26 | 484 | Two collections PABA and self‐report used to assess completenessTotal excretion divided by 0.86a | FFQ, past 12 mo: single Diet History Questionnaire developed and evaluated at the National Cancer Institute | No | No | Bias, attenuation factor of 0.11 (95% CI, 0.00–0.23), correlation adjusted for within‐person biomarker variation for pooled data | |
| Freedman, 2015/AMPM validation study18, 27 | 465 | Two collections, self‐report, volume and creatinine excretion used to assess completenessTotal excretion divided by 0.86a | FFQ, past 12 moA single FFQ (Harvard) was administered 1 to 14 mo after the beginning of the study | N/S | No | Bias, attenuation factor of 0.10 (95% CI, 0.00–0.21), correlation adjusted for within‐person biomarker variation for pooled data | |
| Freedman, 2015/Energetics Study 2006–200918, 28 | 263 | Two collections, PABA used to assess completenessTotal excretion divided by 0.86a | FFQ, past 12 moAdministered onceNational Cancer Institute Diet History Questionnaire | No | N/S | Bias, attenuation factor of −0.05 (95% CI, −0.17 to 0.08), correlation adjusted for within‐person biomarker variation for pooled data | |
| Freedman, 2015/NPAAS 2007–200918, 29 | 450 | Single collectionTotal excretion divided by 0.86a | FFQ, past 3 moWHI FFQ and the nutrient database Nutrition Data System for Research, University of Minnesota was used to analyze the results | N/S | N/S | Bias, attenuation factor of 0.08 (95% CI, 0.00–0.17), correlation adjusted for within‐person biomarker variation for pooled data | |
| Hsu‐Hage, 1992/Melbourne Chinese Health Study30 | 97 | Single collection | FFQ, past 12 mo, adapted from CSIRO FFQ for Melbourne Chinese to assess usual intake (110 items)Portion sizes and nutrient intakes were estimated using the 1990 Australian Food Composition Tables | N/S | N/S | N/S | |
| Kelly, 2015/Food Choice at Work study31 | 50 | Single collection, PABA used to assess completeness | FFQ version of EPIC (150 food items) adapted for Irish population, past 12 mo, nutrient values from Food Standards Agency and McCance and Widdowson's Food Composition Tables | No | N/S | Bland‐Altman, mean difference, 9.1 (95% CI, −5.7 to 24) mmol/d; 95% mean difference, −95.7 to 113.9; AUC, 0.76 (95% CI, 0.6–0.9) | |
| Lassale, 200932 | 62 | Two collections, creatinine excretion used to assess completeness | CSIRO Australia FFQ developed in the 1980s and subsequently evaluated in 1991, designed to estimate usual food and drink intake during the past 12 mo, with nutrient composition derived from four sources: the Australian nutrient database, British Food Composition Tables, USDA food tables, and manufacturers’ data | N/S | No | Correlation (0.352) (P < 0.01); agreement in rankings by quintile; weighted κ 0.31 (quintiles of distribution); regression analysis | |
| Li, 201433 | 964 | Single collection, PABA used to assess completeness | FFQ, N/S | Yes | N/S | Correlation (0.07); mean difference; proportion underestimated and overestimated | |
| Murikami, 2012/Japanese Dietetic Students’ Study for Nutrition and Biomarkers20 | 1043 | Single collection, creatinine excretion used to assess completeness | 150 items, past 1 moStandard Tables of Food Composition in Japan used | Yes | No | Ratio of FFQ to 24‐h urine values (mean, 1.10; SD, 0.70 significantly different from 1.0); also by energy intake (under‐reporters, acceptable, and over‐reporters of energy intake) | |
| Perin, 201334 | 108 | Single collection | Sodium‐specific FFQ (15 foods), past 12 mo | No | N/S | Mean and medians stated only | |
| Sasaki, 2003/JPHC study)35 | 89 | Two collections, PABA used to assess completeness | FFQ (138 items), past 12 mo | N/S | Yes. | Correlation (men 0.24, women −0.10) | |
| Sasaki, 199836 | 223 | Single collection, creatinine excretion used to assess completeness | Diet history (FFQ, 138 items), preceding mo | Yes (“seasonings”) | No | Urinary dietary ratio (mmol); mean (SD) men 0.97 (0.66), women 0.84 (0.46): correlation for log transformed data (men 0.09, women 0.16); adjustedb correlation (men 0.14, women 0.23) | |
| Trijsburg, 2015/DuPLO study37 | 198 | Two collections, PABA used to assess completeness | Two FFQ (180 items) 7 mo apart, past mo | No | No | Bias (%) (reference urine): −41.6 (underestimate), P < 0.01 |
Abbreviations: AMPM, Automated Multiple‐Pass Method; AUC, area under the curve; CI, confidence interval; CSIRO, Commonwealth Scientific and Industrial Research Organisation; EPIC, European Prospective Investigation Into Cancer and Nutrition; FFQ, food frequency questionnaire; JPHC, Japan Public Health Center‐Based Prospective Study on Cancer and Cardiovascular Diseases; NPAAS, Nutrition and Physical Activity Assessment Study of Women's Health Initiative Observational Study; N/S, not stated; OPEN, Observing Protein and Energy Nutrition; PABA, para‐amino benzoic acid; SD, standard deviation.
Consumption estimated assuming 86% of ingested sodium excreted in the urine.
Adjusted for total energy intake and urinary creatinine excretion.
As this review is exploratory in nature, no formal risk of bias assessment was performed. All sodium consumption data are expressed in milligrams of sodium per day using the following conversions: 1 mmol Na = 1 mEq Na = 23 mg Na and 1 g Na = 2.54 g NaCl.
3. RESULTS
The initial search of databases identified 503 articles, and 25 articles were identified from other sources (colleagues and networks, article reference lists, and an updated search in November 2016) (Figure). After 70 duplicates were removed, 458 titles and abstracts were screened, and 108 full‐text articles were assessed for eligibility. One publication18 included results from five studies and several studies included several measures of dietary assessment (eg, FFQ and 24‐hour diet recall). Eighteen studies included assessment by FFQ and 24‐hour urine collection in the same participants and are included in this review. Information on authors, sample size, 24‐hour urine, dietary assessment, accounting for discretionary salt, assessments concurrent (or not), and methods of comparison are described in Table 1. More detail for each study is contained in Appendix C. Of the 18 studies, 16 are described as validation studies, one as a cross‐sectional study, and one as a cohort study. Five of the studies were conducted in the United States, four in Japan, two in Australia, one in Brazil, and one each in Canada, China, Ireland, South Africa, the United Kingdom, and the Netherlands. Sample sizes of those included in the analysis range from 50 to 1043 participants, with results from a total of 5681 participants across all studies. Four of the studies included women only, and 14 studies included both men and women. Seven studies reported including healthy participants and excluded participants with listed medical conditions, four studies included participants with hypertension, and seven studies did not state whether participants were included or excluded on the basis of any medical conditions.
Figure 1.
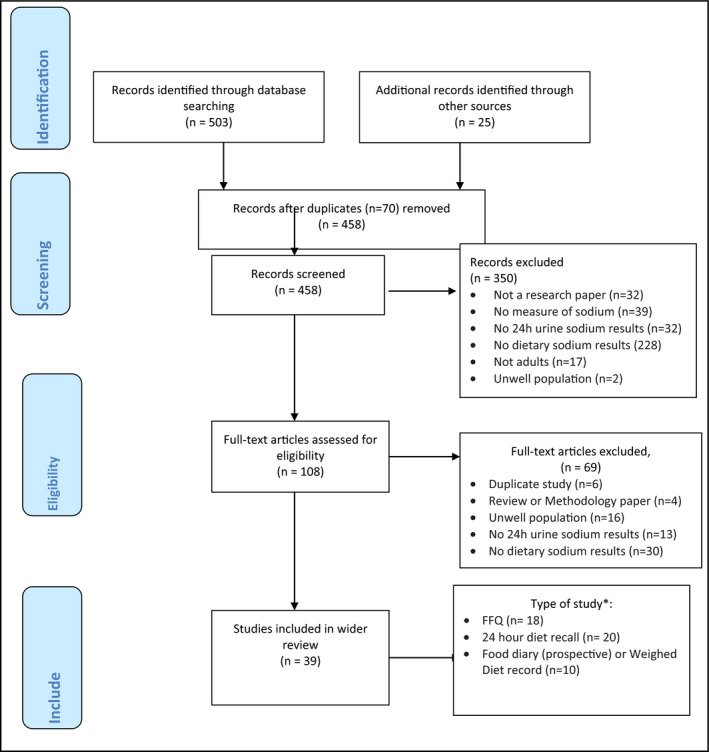
PRISMA flow diagram search strategy. *Some studies included several methods of dietary assessment. FFQ indicates food frequency questionnaire
Twelve studies described a method used to evaluate the completeness of 24‐hour urine collections: four studies used para‐amino benzoic acid (PABA); four studies used an assessment of urine creatinine excretion; and four studies used a combination of methods including urine volume, self‐reported missing urine collections, PABA, and creatinine excretion. The methods used to interpret 24‐hour urinary creatinine and PABA excretion, and therefore which urine samples were likely to be incomplete, varied between studies. For example the PABA excretion cutoff levels for determination of complete urine included >75% PABA urinary recovery, 70% to 103% PABA recovery,31 ≥85% PABA recovery,38 ≥78% PABA recovery,37 and 85% to 110% PABA recovery.18, 25 One study reported that collections with <70% PABA recovery were excluded, and those with 70% to 85% recovery had sodium content adjusted to 93% PABA recovery.18, 39 Methods of assessment of incomplete samples using creatinine excretion included an assessment of within‐ and between‐subject variability and exclusion of samples where creatinine (mmol)/body weight (kg) was outside a certain range (different for men and women).
There was variability in the number of 24‐hour urine collections per participant: 10 studies included a single collection, six studies included two collections, one study included three collections, and one study included six collections. Three studies collected 24‐hour urine samples in the period during which the FFQ was completed, nine collected urine at a different time, and six did not specify whether the FFQ and urine collections were concurrent. When reporting urinary sodium excretion results, five studies divided the 24‐hour urine results by 0.86 to account for incomplete excretion of dietary sodium in urine.18, 40 All studies reported estimates from the dietary assessment and the 24‐hour urine as mean and standard deviation (n = 11), mean and 95% confidence interval (n = 2), or geometric mean and 95% confidence interval (n = 5, all from a single publication).18
Three of the FFQs were specifically designed to assess sodium intake. Charlton and colleagues22 compared results from a sodium‐specific FFQ with 42 items examined over 7 days with those of three 24‐hour urine collections (correlation of 0.173); Ferrera‐Sae and colleagues24 compared results from a sodium‐specific FFQ with 44 items over a 12‐month period with a single 24‐hour urine collection (correlation coefficient stated as not significant); and Perin and colleagues34 included results from a sodium‐specific FFQ with 15 foods over 12 months and those from a single 24‐hour urine collection (correlation not reported). Eleven other FFQs estimated intakes over the previous 12 months, two were for a 3‐month period, three for 1 month, and one over the previous 7 days. One study did not report the time period for assessment. The number of items on the FFQs ranged from 1534 to 150,31 and not all included estimates of discretionary salt added in the home.
As the majority of studies included are validation studies (n = 16), most reported the results of statistical comparison between methods, including correlation, regression, agreement using Bland‐Altman methods,41 ratio of estimates, and κ. Most studies reported on correlation coefficients (Pearson or Spearman). Correlation coefficients reported in this review were generally low, ranging from those reported as not statistically significant (Farreira‐Sae and colleagues24) to r = 0.36 (Day and colleagues23). The correlation coefficients for the studies that reported concurrent data collection (Charlton and colleagues, r = 0.173; Day and colleagues, r = 0.36; and Sasaki and colleagues, r = 0.24 for men and −0.10 for women) do not show substantially higher correlation coefficients than nonconcurrent assessments. Charlton and colleagues22 assessed the extent to which a sodium‐specific FFQ was able to accurately categorize intake in individuals into high (>2300 mg/d) or low, (κ = 0.0318), and Lassale and colleagues32 assessed quintiles of intake (weighted κ = 0.31). The strength of agreement demonstrated by these κ statistics were described as only poor to fair.42 Only one study reported using the Bland‐Altman method to test agreement between estimates of sodium intake from the FFQ and 24‐hour urine. Kelly and colleagues31 compared results of a single 24‐hour urine collection and a version of the EPIC (European Prospective Investigation Into Cancer and Nutrition) FFQ adapted for the Irish population among 50 volunteers. The mean (standard deviation) difference between the two methods was 209 (1205) mg/d and the 95% limits of agreement were 2201 (2620) mg/d, indicating poor agreement between the two measures. There was no obvious bias at low or high sodium intakes for the comparisons (n = 50).
Freedman and colleagues18 reported on a pooled analysis from five validation studies: the Nutrition Biomarker Study for the Women's Health Initiative,25, 43 the OPEN (Observing Protein and Energy Nutrition) study,26 the AMPM (Automated Multiple‐Pass Method) validation study,27, 44 the Energetics Study,28 and the NPAAS (Nutrition and Physical Activity Assessment Study of the Women's Health Initiative Observational Study).29, 43 None of the FFQs in this analysis were sodium specific, and all had limited ability to quantify discretionary salt intake. In the pooled analysis, authors report that sodium intake assessed by FFQ was on average 30% less than that measured in 24‐hour urine collections (ranging from an underestimate of 2% among women in the Energetics Study, to a 52% underestimate among men in the AMPM study). Bias in sodium density (energy adjusted) was more variable, and ranged from an average overestimate of approximately 30% in the Energetics Study, and an average underestimate of approximately 30% in the AMPM study. Underreporting of intake relating to sodium intake was associated with high body mass index, lower education, being male, and being black. There was a low correlation between the sodium intake assessed by FFQ and urinary sodium excretion of 0.16 (0.17 for men and 0.15 for women) in the pooled analysis, although the correlation was higher (approximately 0.32) for sodium density. The authors also calculated attenuation factors for sodium for each study (Table 1), and reported a pooled average attenuation factor of 0.08 (95% confidence interval, 0.04–0.13).18
4. DISCUSSION
We found poor agreement between dietary sodium estimation by FFQ and 24‐hour urinary excretion in 18 reported studies. These were assessed by several statistical methods including correlation and comparison of mean estimates. Reported correlations were generally low for studies that compared 24‐hour urine with FFQ results. One study reported mean differences using the Bland‐Altman method; the 95% limits of agreement were wide for this study, suggesting poor agreement in individual cases. There was considerable variability between studies on methods of data collection, including number of 24‐hour urine samples collected, assessment of urine for complete collection, and FFQ design (length of time assessed and number of items, and assessment of discretionary salt intake). This variation made direct comparison of FFQs in this review impractical, suggesting that guidance for validation studies of sodium intake assessment is needed.
For epidemiological studies (particularly cohort studies) and clinical trials, where an estimate of individual usual intake is linked with clinical outcomes in that individual (even though group level measures of association are presented), a high level of measurement validity is required. FFQs are widely used because they estimate usual intake (over a specified period) in a single encounter. FFQs that assess intake over 1 month will account for day‐to‐day variability but not seasonal variation. A questionnaire that asks participants about their intake over a 12‐month period will account for seasonal variation, but may be more prone to recall bias, resulting in a higher degree of measurement error.45 Variability in the design of specific FFQs is appropriate, given that FFQs need to be relevant to local dietary patterns, habits, and food availability and matched to appropriate local food composition databases in order to be reliable. The additional benefit of FFQs is that they can be used to measure multiple exposures, in the form of nutrients, foods, or dietary patterns, although sodium‐specific FFQs have been designed.22, 24, 34 However, based on this review, current FFQs are not good measures of usual sodium intake.
Correlations between FFQ and 24‐hour urine sodium reported in this review were generally low, ranging from those reported as not statistically significant24 to 0.36.23 Values of correlation between self‐reported and measured intake <0.4 in nutritional validation studies are regarded as “undesirable”18 and such values might be regarded as poor according to many interpretations of correlation coefficients,46 suggesting relatively poor individual‐level validity. Mean correlation coefficients from a different review of FFQs estimating intakes of other nutrients and relevant biomarkers varied between 0.35 (for vitamin A) to 0.54 (for fat).9 Only one study reported using the Bland‐Altman mean difference method to test agreement between the two methods.
One study examined the extent to which an FFQ can correctly assign intakes in individuals into groups of higher vs lower sodium,22 and one examined results in quintiles.32 In epidemiological studies, valid assignment of individuals’ intake to high or low (or groups such as tertiles) may be acceptable if an accurate measure of usual intake is not possible. However, it will be important to understand the degree to which the FFQ instrument is both reliable and valid in assigning individuals to appropriate groups in interpretation of results of such studies. κ is often used to assess the strength of agreement in such studies. An alternative approach has been taken by Freedman and colleagues, who examined bias in different groups and calculated attenuation factors for FFQs, which can be used in epidemiological studies to account for systematic errors in estimates of sodium intake.18 Attenuation factors are a way of adjusting relative risk estimates from a cohort study (examining the association between nutrient intake and disease outcomes) to allow for the bias inherent in dietary measurements. This bias means that the effect of certain factors is “attenuated,” or made closer to 1. The smaller the estimated attenuation factor, the greater the bias towards 1 of the relative risk estimate.22 For example, if the true relative risk is 2.68 and the attenuation factor is 0.03, the estimated relative risk would be 2.680.03 = 1.03. However, if the true relative risk is only 1.025 but the attenuation factor is 1.2, the estimated relative risk would still be 1.03.16, 39 An alternative approach suggested by some authors, which has not been tested by any of the studies in this review, is to use a combination of biomarkers and a FFQ to optimize the validity of assessment; however, this is only useful if the FFQ is valid. With this approach, a range of measures could be used to estimate usual intake, including 24‐hour urine (biomarker), FFQ, and possibly anthropometric information.12, 47 Further research is needed to test the validity and reliability of this method for dietary sodium assessment.
Clearly, validation studies of specific FFQ designs to estimate sodium intake are essential, and it is important that validation is not viewed as a dichotomous outcome. “Validity usually is a matter of degree rather than an all‐or‐none property, and a validation is an unending process.”48 It is, therefore, not enough to describe an FFQ as a “validated questionnaire” but rather a description of the extent to which the instrument was found to be reliable or valid. A description of the degree to which individuals are correctly assigned to groups of levels of sodium intake is also required if analysis by group is used. We recommend greater consistency of methods for validation studies of FFQs for sodium intake. A review of published validation studies of FFQs in 20049 and an accompanying consensus statement49 include a series of recommendations, including that all FFQs should be validated for reproducibility and accuracy in a sample of participants from the relevant population. The authors recommend that FFQs be compared with results from suitable reference methods (such as 24‐hour recall or diet records or relevant biomarkers), and that multiple days of data collection using the reference method be undertaken over a similar period of assessment as the FFQ. They recommend that the Bland‐Altman method be used to measure agreement between the two methods. Correlation is commonly used in this context, but it should be noted that it is not a good measure of calibration or agreement, instead it measures whether two measures are linearly related. The authors suggest that correlation or regression may be used in conjunction with the Bland‐Altman method. κ and measures of sensitivity and specificity are suggested as appropriate statistical methods if the results of the FFQ are to be used as categorical variables.9 With these recommendations in mind, we suggest (see Table 2) that for assessment of dietary sodium intake, estimates of usual intake from FFQs be compared with results from multiple days of 24‐hour urine collection (at least two and up to seven), over a similar period of assessment as the FFQ. A single 24‐hour urine collection is likely to be inadequate for validation studies of FFQs, as at least two or three 24‐hour urine collections, preferably several months apart, are required to estimate usual intake.7 Twenty‐four‐hour urine collections should be assessed for completeness using a suitable method (such as PABA).50 We also recommend that the Bland‐Altman method be used to measure agreement between the two methods. Regression and/or correlation may be used to support Bland‐Altman methods. κ and measures of sensitivity and specificity are appropriate if sodium intake is to be reported as a categorical or binary variable.9
Table 2.
Recommendations for validation studies of FFQs measuring sodium intake based on Cade and colleagues9
| Food Frequency Questionnaire |
| FFQs should be validated in population of interest, with reference to regularly updated food composition databases that relate to local food supplies |
| FFQs should include an estimate of discretionary salt used (in cooking or at the table) |
| Reference method: 24‐h urine |
| 24‐h urinary sodium excretion is the recommended reference method |
| At least two and up to seven 24‐h urine collections per participant should be collected |
| Urine collections should be undertaken over a similar period of assessment as the FFQ |
| 24‐h urine collections should be assessed for completeness using a suitable method (such as PABA excretion) |
| Statistical analysis |
| Multiple methods should be used, depending on the purpose of research |
| Group means should be considered for studies where an assessment of population mean is the outcome of interest |
| For epidemiological studies, Bland‐Altman methods should be used to assess agreement between sodium estimates from FFQs and urinary excretion |
| Additional useful statistical methods include correlation, regression, and κ if data are to be presented as categorical or binary |
| Relative bias should be considered (eg, at high or low intakes or in different population subgroups) |
| Sample size should be carefully considered–at least 50 to 100 participants–for each population group has been suggested |
| Reporting |
| Details of results of validation studies should be reported in utilization studies, rather than describing the FFQ as a “validated questionnaire” |
Abbreviations: FFQ, food frequency questionnaire; PABA, para‐amino benzoic acid.
Validation studies should also report the degree to which measurement error has occurred. The degree to which this measurement error is differential (systematic) or nondifferential (random) is important in the interpretation of epidemiological studies. Nondifferential measurement error tends to bias measures of association towards the null, resulting in an underestimation of the degree to which sodium intake is associated with clinical outcomes. The analysis presented in Freedman and colleagues suggests that measurement error was differential, in that under‐reporting of intake relating to sodium intake was associated with high body mass index, lower education, being male, and being black.18 Other studies have also suggested that dietary assessment by FFQs includes both differential and nondifferential measurement error, resulting in an unclear effect on subsequent measures of association.17, 51
5. CONCLUSIONS
Current FFQs should not be used in research to assess sodium intake. Our results show generally poor agreement between dietary sodium estimation by current FFQs and 24‐hour urinary excretion. Such limitations associated with nutritional assessment by FFQ are not unusual and have led some to question the ongoing use of FFQs in epidemiological studies.11 Standardization of validation studies for FFQs used to assess sodium intake is needed. Repeated 24‐hour urine collections remain the gold standard for sodium intake assessment.
CONFLICT OF INTEREST
NC is a member of World Action on Salt and Health (a dietary salt reduction organization) and is a paid consultant for the Novartis Foundation, which involves travel expenses and personal fees for site visits; received a one‐time contract (2016) to develop a survey; and was a paid member of an advisory board for Midmark in 2017. The other authors have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
This research was funded by a grant from the University of Otago. The authors would like to thank the members of the TRUE Consortium expert committee for helping design the analytic program and need for this meta‐analysis as well as reviewing and commenting on this article. The members of the TRUE sodium expert committee are Drs Cheryl Anderson, Larry Appel, JoAnne Arcand, Norm Campbell (Chair), Francesco Cappuccio, Mary Cogswell, Nancy Cook, Paul Elliott, Fengjun He, Antti Jula, Mary L'Abbe, Daniel Lackland, Graham MacGregor, Rachael McLean, Doreen Rabi, Paul Whelton, and Mark Woodward. At a later date, the scientific committee will consider developing recommendations on the basis of this analysis and others.
Appendix A: Search Terms
Aovid Medline 1946 To Present With Daily Update
.(Dietary sodium.mp or exp Sodium, Dietary/ or Sodium Chloride, Dietary/ or Sodium, Dietary/ or Diet, Sodium Restricted/)
(24 hr* or 24 hour* or 24‐hr* or 24 hour*)
(urin*)
(Energy Intake/ or Questionnaires/ or Diet Surveys/ or nutrition Surveys/ or Diet Records/ or Food Habits/ or Adult/ or Nutrition Assessment/ or Diet/ or dietary assessment.mp or Middle Aged/))
2 and 3
1 and 5
4 and 6
limit 7 to humans
Cinharl Via Ebscohost
(MH “Diet, Sodium‐Restricted”) OR (MH “Sodium Chloride”) OR (MH “Dietary Reference Intakes”) OR (MH “Food Intake”) OR (MH “Sodium Dietary”) OR (MH “Sodium Chloride Dietary”) OR (MH “Nutritional Status: Nutrient Intake (Iowa NOC)”)
(MH “24‐hour Urine Collection”) OR (MH “Urine Specimen Collection”)
(MH “Nutritional Assessment”)
1 and 2 and 3
Embase Via Ovid
(dietary sodium.mp or sodium intake/ or sodium chloride/)
(urine or urinalysis)
(24 hour.mp or urine/ or 24‐hr*.mp or 24 hour*.mp or 24 hr*.mp)
(dietary assessment.mp or nutritional assessment/ or diet/th [Therapy])
(sodium urine level/ or kidney/ or sodium excretion/ or sodium excretion/ or urine/)
2 or 3
1 or 6
5 or 6
1 and 8
4 and 9
Lilacs
tw: (sodium urine) AND (instance: “regional”) AND (db (“LILACS”))
Cochrane Library
(salt sodium dietary)
Google Scholar
(salt sodium dietary 24 hour urine)
Appendix B.
B.1.
Author: TRUE ConsortiumABCDEFGHIJ
AAmerican Heart Association.
BBritish and Irish Hypertension Society.
CChinese Regional Office of the World Hypertension League.
DHypertension Canada.
EInternational Council of Cardiovascular Prevention and Rehabilitation.
FInternational Society of Hypertension.
GInternational Society of Nephrology.
HPan American Health Organization/World Health Organization Technical Advisory Group on Cardiovascular Diseases Prevention Through Population Wide Dietary Salt Reduction.
IWorld Hypertension League.
JWorld Stroke Organization.
Appendix C: Characteristics of included studies
C:.1.
Table Characteristics of included studies describing results of estimated sodium intake from FFQ and 24 hour urinary excretion
| First author, y/Name of Study | Type of study | Participants and setting | No. (final analysis) | 24‐h Urine collection validated for completeness | 24‐h Urinary sodium resultsb (including No. of collections/participant) | Dietary assessment (FFQ) | Discretionary salt accounted for | FFQ sodium resultsb | 24‐h Urine and FFQ concurrent | Method(s) of comparison | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bedford, 2011 | Cohort | Healthy volunteers aged 19–35 y (100% women)Canada | 102 | N/S | Single collectionMean, 2942 (SD, 1062) mg/d | FFQ (National Cancer Institute) 124 items, past 12 mo, analyzed with a Canadian version of the nutrient database | No | Mean, 2648 (SD, 1089) mg sodium | No | Correlation (0.21) | |
| Charlton, 2008 | Validation | Convenience sample of 180 adults with hypertension and 145 adults with normotension (51% women) aged 20–65 ySouth Africa | 284 | PABA, urine volume, and urinary creatinine concentration | Three collections by tertiles of intake: mean urinary excretion, 3049 (SD, 1182), 3514 (SD, 1659), and 3670 (SD, 2039) mg/d | Salt‐specific FFQ (42 items), previous 7 d | Yes | By ethnicity: mean, 1211 (SD, 641), 1853 (SD, 589), and 1873 (SD, 663) mg/d for black, mixed ancestry, and white ethnic groups, respectively | Yes | Correlation (0.173); κ (0.0318) categorizing high (>2400 mg/d) vs low (<2400 mg/d) sodium intake | |
| Day, 2001/EPIC‐Norfolk cohort study | Validation | Volunteers aged 45–74 y (sex and disease status N/S)United Kingdom | 123 | PABA | Six collections: mean, 3335 mg/d (SD, 1297) Coefficient of variation 0.39 | Two FFQs (130 items) 18 mo apartFFQ self‐administered, past 12 moModified questionnaire from US Nurses’ Health StudyCalculation based on Ministry of Agriculture, Fisheries, and Food composition tables | N/S | Mean, 2766 (SD, 1074) mg/d sodium | Yes | Sample variance; correlation (0.36); regression analysis | |
| Ferreira‐Sae, 2009 | Validation | Patients with hypertension aged 18–85 y (63% women)Brazil | 132 | N/S | Single collectionTotal excretion divided by 0.86aMean, 5384 (SD, 2402) mg/d | Salt‐specific FFQ 44 items, past 12 moNutwin database software developed by Federal University of Sao Paulo | In a separate questionnaire | Including estimates of discretionary salt: mean, 5093 (SD, 267) mg/d | No | Correlation | |
| Freedman, 2015/Nutrition Biomarker Study for WHI 2004–2005 | Validation | Representative healthy sample from WHI dietary modification trial (100% women) Mean age, 70.9 yUnited States | 544 | PABA and self‐reported missing collections | Single collectionTotal excretion divided by 0.86a Geometric mean, 3263 (95% CI, 3155–3373) mg/d | WHI FFQ, past 3 mo, nutrient databaseNutrition Data System for Research, University of Minnesota to analyze the results | N/S | Geometric mean, 2188 (95% CI, 2088–2293) mg/d | No | Bias; attenuation factor of 0.12 (95% CI, 0.03–0.20); correlation adjusted for within‐person biomarker variation for pooled data | Pooled data from five studiesAverage correlation 0.16 (men r = 0.17, women r = 0.15) |
| Freedman, 2015/OPEN study 1999–2000 | Validation | Random sample of healthy participants in the OPEN study aged 40–69 y (46% women)United States | 484 | PABA and self‐reported lost specimens | Two collectionsTotal excretion divided by 0.86a Geometric mean for men, 4502 (95% CI, 4287–4727) mg/d; for women, 3310 (3126–3503) mg/d | FFQ, past 12 moSingle Diet History Questionnaire developed and evaluated at the National Cancer Institute | No | Geometric mean, men 3070 (95% CI, 2920–3227) mg/d; women 2308, (95% CI, 2186–2436) mg/d | No | Bias; attenuation factor of 0.11 (95% CI, 0.00–0.23); correlation adjusted for within‐person biomarker variation for pooled data | |
| Freedman, 2015/AMPM validation study | Validation | Healthy volunteers from AMPM study aged 30–69 y (50% women)United States | 465 | Self‐reported missing collections, volume, and creatinine excretion | Two collectionsTotal excretion divided by 0.86a Geometric mean for men, 4648 (95% CI, 4421–4886) mg/d; for women, 3494 (95% CI, 3330–3666) mg/d | FFQ, past 12 moA single FFQ (Harvard) was administered 1–14 mo after the beginning of the study | N/S | Geometric mean for men, 2188 (95% CI, 2088–2293) mg/d; for women, 1851 (95% CI, 1762–1945) mg/d | No | Bias; attenuation factor of 0.10 (95% CI, 0.00–0.21); correlation adjusted for within‐person biomarker variation for pooled data | |
| Freedman, 2015/Energetics Study 2006–2009 | Validation | Healthy volunteers from Energetics Study aged 21–69 y (64% women)United States | 263 | PABA | Two collectionsTotal excretion divided by 0.86a Geometric mean for men, 3692 (95% CI, 3371–4043) mg/d; for women, 2555 (95% CI, 2345–2783) mg/d | FFQ, past 12 mo,Administered onceNational Cancer Institute Diet History Questionnaire | No | Geometric mean for men, 3377 (95% CI, 3077–3706) mg/d; for women, 2459 (95% CI, 2270–2662) mg/d | N/S | Bias; attenuation factor of −0.05 (95% CI, −0.17 to 0.08); correlation adjusted for within‐person biomarker variation for pooled data | |
| Freedman, 2015/NPAAS 2007–2009 | Validation | Representative sample of healthy participants (mean age, 70.5 y; 100% women) from WHI Observational StudyUnited States | 450 | N/S | Single 24‐h urine collectionUrinary sodium values were divided by 0.86a Geometric mean for women, 3056 (95% CI, 2933–3183) mg/d | FFQ, past 3 mo,WHI FFQ and the nutrient databaseNutrition Data System for Research, University of Minnesota was used to analyze the results | N/S | Geometric mean sodium intake, 2383 (95% CI, 2286–2484) mg/d | N/S | Bias; attenuation factor of 0.08 (95% CI, 0.00–0.17); correlation adjusted for within‐person biomarker variation for pooled data | |
| Hsu‐Hage, 1992/Melbourne Chinese Health Study | Validation | Convenience sample. (sex, age, and disease status N/S)Australia | 97 | N/S | Single collectionMean for men, 4163 mg/d (SD, 1978) and for women, 3542 mg/d (SD, 1702) | FFQ, past 12 mo, adapted from CSIRO FFQ for Melbourne Chinese to assess usual intake (110 items)Portion sizes were estimated and nutrient intakes were estimated using the 1990 Australian Food Composition Tables | N/S | Mean for men, 1334 mg/d sodium and for women, 1196 mg/d sodium | N/S | N/S | |
| Kelly, 2015/Food Choice at Work Study | Validation | Volunteers aged 18–64 y; 36% women, 12% with hypertension)Ireland | 50 | PABA | Single collectionMean, 3174 (SD, 1219) mg/d | FFQ version of EPIC (150 food items) adapted for Irish population, past 12 moNutrient values from Food Standards Agency and McCance and Widdowson's Food Composition Tables | No | Mean, 2967 (SD, 1150) mg/d sodium | N/S | Bland‐Altman, mean difference, 9.1 (95% CI, −5.7 to 24) mmol/d; 95% mean difference, −95.7 to 113.9; AUC, 0.76 (95% CI, 0.6–0.9) | |
| Lassale, 2009 | Validation | Healthy volunteers aged 30–60 y (100% women)Australia | 62 | Creatinine | Two collectionsMean, 2921 (SD, 989) mg/d | CSIRO Australia FFQ developed in the 1980s and subsequently evaluated in 1991, designed to estimate usual food and drink intake over past 12 moWith nutrient composition derived from four sources: the Australian nutrient database, British Food Composition Tables, USDA food tables, and manufacturers’ data | N/S | Mean, 3008 (SD, 1186) mg/d | No | Correlation (0.352) (P < 0.01), agreement in rankings by quintile, weighted κ = 0.31 (quintiles of distribution), and regression analysis | |
| Li, 2014 | Validation | Random sample aged 18–69 y (48% women)Disease status: N/SChina | 964 | N/S | Single collectionMean for men, 5709 (95% CI, 5354–6024) mg/d and for women, 5315 (95% CI, 5000–5591) mg/d | FFQ NS | Yes | Mean for men, 4291 (SD, 3819–4343) and for women, 4016 (SD, 3740–4331) mg/d | N/S | Correlation (0.07), mean difference, and proportion underestimated and overestimated | |
| Murikami, 2012/Japanese Dietetic Students’ Study for Nutrition and Biomarkers | Validation | Volunteers aged 18–22 y (100% women) Disease status N/SJapan | 1043 | Urinary creatinine excretion | Single collectionTotal excretion divided by 0.86a Mean, 3869 (SD, 1491) mg/d | 150 items, past 1 moStandard Tables of Food Composition in Japan | Yes | Mean, 3629 (SD, 1225) mg/d | No | Ratio of FFQ to 24‐h urine values (mean, 1.10; SD, 0.70 significantly different from 1.0); also by energy intake (under‐reporters, acceptable, and over‐reporters of energy intake) | |
| Perin, 2013 | Cross‐sectional | Patients with hypertension, mean age 56.7 y, (52% women)Japan | 108 | Not stated | Single collectionMean, 4814 (SD, 2300) mg/d | Sodium‐specific FFQ (15 foods), past 12 mo | No | Mean, 984 (SD, 1063) mg/d | N/S | Mean and medians stated only | |
| Sasaki, 2003/JPHC study | Validation | Volunteers from representative sample (64% women), age and disease status not statedJapan | 89 | Creatinine excretionResults were no different, so present results with all patients | Two collectionsMean for men, 4669 (SD, 1978) and for women, 4600 (SD, 1702) mg/d | FFQ (138 items), past 12 mo | N/S | Mean for men, 6026 (SD, 2829) and for women, 6026 (SD, 2714) mg/d | Yes | Correlation (men 0.24, women −0.10) | |
| Sasaki, 1998 | Validation | Volunteers (31% women) age and disease status not statedJapan | 223 | Creatinine excretion | Single collectionMean for men, 3795 (SD, 1242) mg/d and for women, 3128 (SD, 1357) mg/d | Diet historyFFQ (138 items), preceding mo | Yes (“seasonings”) | Mean for men, 4508 (SD, 1610) and for women, 4117 (SD, 1357) mg/d sodium | No | Urinary dietary ratio (mmol): mean for men, 0.97 (SD, 0.66) and for women, 0.84 (SD, 0.46); correlation for log‐transformed data (men 0.09, women 0.16); adjustedc correlation (men 0.14, women 0.23) | |
| Trijsburg, 2015/DuPLO study | Validation | Random sample from DuPLO study aged 20–70 y (54% women)Disease status N/SThe Netherlands | 198 | PABA | Two collections. Mean, 3983 (SD, 1264) mg (n = 197) | Two FFQ (180 items), 7 mo apart–past mo | No | Mean sodium intake, 2137 (SD, 708) mg/d | No | Bias (%) (reference urine): −41.6 (underestimate), P < 0.01 | |
Abbreviations: AMPM, Automated Multiple‐Pass Method; AUC, area under the curve; CI, confidence interval; CSIRO, Commonwealth Scientific and Industrial Research Organisation; EPIC, European Prospective Investigation Into Cancer and Nutrition; FFQ, food frequency questionnaire; JPHC, Japan Public Health Center‐Based Prospective Study on Cancer and Cardiovascular Diseases; NPAAS, Nutrition and Physical Activity Assessment Study; N/S, not stated; OPEN, Observing Protein and Energy Nutrition; PABA, para‐amino benzoic acid; SD, standard deviation; USDA, United States Department of Agriculture; WHI, Women's Health Initiative.a Consumption estimated assuming 86% of ingested sodium excreted in the urine. b Combined mean unless results only presented by sub‐group. c Adjusted for total energy intake and urinary creatinine excretion.
McLean RM, Farmer VL, Nettleton A, et al. Assessment of dietary sodium intake using a food frequency questionnaire and 24‐hour urinary sodium excretion: a systematic literature review. J Clin Hypertens. 2017;19:1214–1230. 10.1111/jch.13148
Funding information
This work was supported by a Univeristy of Otago Research Grant
REFERENCES
- 1. Webster J, Waqanivalu T, Arcand J, et al. Understanding the science that supports population‐wide salt reduction programs. J Clin Hypertens (Greenwich). 2017;19:569‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cappuccio FP, Campbell NR. Population dietary salt reduction and the risk of cardiovascular disease: a commentary on recent evidence. J Clin Hypertens (Greenwich). 2017;19:4‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell NR. Dissidents and dietary sodium: concerns about the commentary by O'Donnell et al. Int J Epidemiol. 2016;46:362‐366. [DOI] [PubMed] [Google Scholar]
- 4. Arcand J, Webster J, Johnson C, et al. Announcing “Up to Date in the Science of Sodium.” J Clin Hypertens (Greenwich). 2016;18:85‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell NR, Lackland DT, Niebylski ML. 2014 dietary salt fact sheet of the World Hypertension League, International Society of Hypertension, Pan American Health Organization technical advisory group on cardiovascular disease prevention through dietary salt reduction, the World Health Organization collaborating centre on population salt reduction, and World Action on Salt & Health. J Clin Hypertens (Greenwich). 2015;17:7‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. TRUE Consortium . Recommended standards for assessing blood pressure in human research where blood pressure or hypertension is a major focus. J Clin Hypertens (Greenwich). 2017;19:108‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun Q, Bertrand KA, Franke AA, Rosner B, Curhan GC, Willett WC. Reproducibility of urinary biomarkers in multiple 24‐h urine samples. Am J Clin Nutr. 2016;105:159‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cogswell ME, Maalouf J, Elliott P, Loria CM, Patel S, Bowman BA. Use of urine biomarkers to assess sodium intake: challenges and opportunities. Annu Rev Nutr. 2015;35:349‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cade J, Burley V, Warm D, Thompson R, Margetts B. Food‐frequency questionnaires: a review of their design, validation and utilisation. Nutr Res Rev. 2004;17:5‐22. [DOI] [PubMed] [Google Scholar]
- 10. Willett W. Nutritional Epidemiology, 3rd edn. Oxford: Oxford University Press; 2013. [Google Scholar]
- 11. Kristal AR, Peters U, Potter JD. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol Biomark Prev. 2005;14:2826‐2828. [DOI] [PubMed] [Google Scholar]
- 12. Willett WC, Hu FB. Not the time to abandon the food frequency questionnaire: point. Cancer Epidemiol Biomark Prev. 2006;15:1757‐1758. [DOI] [PubMed] [Google Scholar]
- 13. Kelemen LE. Food frequency questionnaires: not irrelevant yet. Cancer Epidemiol Biomark Prev. 2006;15:1054. [DOI] [PubMed] [Google Scholar]
- 14. Strazzullo P, D'Elia L, Kandala N, Cappuccio F. Salt intake, stroke, and cardiovascular disease: meta‐analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawkes C, Webster J. National approaches to monitoring population salt intake: a trade‐off between accuracy and practicality? PLoS One. 2012;7:e46727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst. 2011;103:1086‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thiebaut AC, Freedman LS, Carroll RJ, Kipnis V. Is it necessary to correct for measurement error in nutritional epidemiology? Ann Intern Med. 2007;146:65‐67. [DOI] [PubMed] [Google Scholar]
- 18. Freedman LS, Commins JM, Moler JE, et al. Pooled results from 5 validation studies of dietary self‐report instruments using recovery biomarkers for potassium and sodium intake. Am J Epidemiol. 2015;181:473‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murakami K, Sasaki S, Takahashi Y, et al. Misreporting of dietary energy, protein, potassium and sodium in relation to body mass index in young Japanese women. Eur J Clin Nutr. 2008;62:111‐118. [DOI] [PubMed] [Google Scholar]
- 20. Murakami K, Sasaki S, Uenishi K, Japan Dietetic Students’ Study for N , Biomarkers G. The degree of misreporting of the energy‐adjusted intake of protein, potassium, and sodium does not differ among under‐, acceptable, and over‐reporters of energy intake. Nutr Res. 2012;32:741‐750. [DOI] [PubMed] [Google Scholar]
- 21. Bedford JL, Barr SI. Higher urinary sodium, a proxy for intake, is associated with increased calcium excretion and lower hip bone density in healthy young women with lower calcium intakes. Nutrients. 2011;3:951‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charlton KE, Steyn K, Levitt NS, Jonathan D, Zulu JV, Nel JH. Development and validation of a short questionnaire to assess sodium intake. Pub Health Nutr. 2008;11:83‐94. [DOI] [PubMed] [Google Scholar]
- 23. Day N, McKeown N, Wong M, Welch A, Bingham S. Epidemiological assessment of diet: a comparison of a 7‐day diary with a food frequency questionnaire using urinary markers of nitrogen, potassium and sodium. Int J Epidemiol. 2001;30:309‐317. [DOI] [PubMed] [Google Scholar]
- 24. Ferreira‐Sae MC, Gallani MC, Nadruz W, et al. Reliability and validity of a semi‐quantitative FFQ for sodium intake in low‐income and low‐literacy Brazilian hypertensive subjects. Pub Health Nutr. 2009;12:2168‐2173. [DOI] [PubMed] [Google Scholar]
- 25. Neuhouser ML, Tinker L, Shaw PA, et al. Use of recovery biomarkers to calibrate nutrient consumption self‐reports in the Women's Health Initiative. Am J Epidemiol. 2008;167:1247‐1259. [DOI] [PubMed] [Google Scholar]
- 26. Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158:1‐13. [DOI] [PubMed] [Google Scholar]
- 27. Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple‐Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324‐332. [DOI] [PubMed] [Google Scholar]
- 28. Arab L, Wesseling‐Perry K, Jardack P, Henry J, Winter A. Eight self‐administered 24‐hour dietary recalls using the Internet are feasible in African Americans and whites: the Energetics Study. J Am Diet Assoc. 2010;110:857‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prentice RL, Mossavar‐Rahmani Y, Huang Y, et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol. 2011;174:591‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsu‐Hage BH, Wahlqvist ML. A food frequency questionnaire for use in Chinese populations and its validation. Asia Pacific J Clin Nutr. 1992;1:211‐223. [PubMed] [Google Scholar]
- 31. Kelly C, Geaney F, Fitzgerald A, Browne G, Perry I. Validation of diet and urinary excretion derived estimates of sodium excretion against 24‐hour urine excretion in a worksite sample. Nutr Metab Cardiovasc Dis. 2015;25:771‐779. [DOI] [PubMed] [Google Scholar]
- 32. Lassale C, Guilbert C, Keogh J, Syrette J, Lange K, Cox D. Estimating food intakes in Australia: validation of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) food frequency questionnaire against weighed dietary intakes. J Hum Nutr Diet. 2009;22:559‐566. [DOI] [PubMed] [Google Scholar]
- 33. Li J, Lu Z, Yan L, et al. Comparison of dietary survey, frequency and 24 hour urinary Na methods in evaluation of salt intake in the population. Chinese J Prevent Med. 2014;48:1093‐1097. [PubMed] [Google Scholar]
- 34. Perin MS, Cornelio ME, Rodrigues RC, Gallani MC. Characterization of salt consumption among hypertensives according to socio‐demographic and clinical factors. Revista Latino‐Americana de Enfermagem. 2013;21:1013‐1021. [DOI] [PubMed] [Google Scholar]
- 35. Sasaki S, Ishihara J, Tsugane S. Validity of a self‐administered food frequency questionnaire in the 5‐year follow‐up survey of the JPHC Study Cohort I to assess sodium and potassium intake: comparison with dietary records and 24‐hour urinary excretion level. J Epidemiol. 2003;13:102‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sasaki S, Yanagibori R, Amano K. Validity of a self‐administered diet history questionnaire for assessment of sodium and potassium: comparison with single 24‐hour urinary excretion. Jpn Circ J. 1998;62:431‐435. [DOI] [PubMed] [Google Scholar]
- 37. Trijsburg L, De Vries JHM, Boshuizen HC, et al. Comparison of duplicate portion and 24 h recall as reference methods for validating a FFQ using urinary markers as the estimate of true intake. Br J Nutr. 2015;114:1304‐1312. [DOI] [PubMed] [Google Scholar]
- 38. McKeown NM, Day NE, Welch AA, et al. Use of biological markers to validate self‐reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort. Am J Clin Nutr. 2001;74:188‐196. [DOI] [PubMed] [Google Scholar]
- 39. Kipnis V, Subar AF, Midthune D, et al. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol. 2003;158:14‐21. [DOI] [PubMed] [Google Scholar]
- 40. Holbrook J, Patterson K, Bodner J, et al. Sodium and potassium intake and balance in adults consuming self‐selected diets. Am J Clin Nutr. 1984;40:786‐793. [DOI] [PubMed] [Google Scholar]
- 41. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307‐310. [PubMed] [Google Scholar]
- 42. Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall; 1991. [Google Scholar]
- 43. Huang Y, Van Horn L, Tinker LF, et al. Measurement error corrected sodium and potassium intake estimation using 24‐hour urinary excretion. Hypertension. 2014;63:238‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rhodes DG, Murayi T, Clemens JC, Baer DJ, Sebastian RS, Moshfegh AJ. The USDA automated multiple‐pass method accurately assesses population sodium intakes. Am J Clin Nutr. 2013;97:958‐964. [DOI] [PubMed] [Google Scholar]
- 45. Smith AF, Jobe JB, Mingay DJ. Retrieval from memory of dietary information. Appl Cogn Psychol. 1991;5:269‐296. [Google Scholar]
- 46. Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284. [Google Scholar]
- 47. Otten JJ, Hellwig JP, Meyers LD. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 48. Kristal AR, Potter JD. Not the time to abandon the food frequency questionnaire: counterpoint. Cancer Epidemiol Biomark Prev. 2006;15:1759. [DOI] [PubMed] [Google Scholar]
- 49. Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food‐frequency questionnaires–a review. Pub Health Nutr. 2002;5:567‐587. [DOI] [PubMed] [Google Scholar]
- 50. John KA, Cogswell ME, Campbell NR, et al. Accuracy and usefulness of select methods for assessing complete collection of 24‐hour urine: a systematic review. J Clin Hypertens (Greenwich). 2016;18:456‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Taren D, Dwyer J, Freedman L, Solomons NW. Dietary assessment methods: where do we go from here? Pub Health Nutr. 2002;5:1001‐1003. [DOI] [PubMed] [Google Scholar]


