Abstract
Fixed‐dose combinations (FDC) have been developed to reduce the pill burden for hypertensive patients. Data on fixed‐dose or free‐dose (freeDC) ramipril/amlodipine (R/A) or candesartan/amlodipine (C/A) combination treatment initiation were assessed. 71 463 patients were prescribed R/A and 10 495 C/A. For both R/A and C/A, FDC patients were younger (both P < .001) and less comorbid. Prior MI (OR: 0.61 and 0.60), prior stroke (OR: 0.68 and 0.70) and CHD (OR: 0.68 and 0.64) were negatively associated with FDC use, whereas hyperlipidemia was positively associated (OR: 1.26 and 1.19). Use of antihypertensive comedication (OR: 0.78; OR: 0.55) and treatment discontinuation within 12 months (HR: 0.65 and 0.82) were less likely in FDC patients, who also showed superior adherence (mean MPR; both P < .001). Cost of the combination was higher for FDCs (both P < .001). FDCs improve persistence and adherence, although they are more commonly prescribed in patients with less cardiovascular disease.
Keywords: adherence, angiotensin receptor blocker, angiotensin‐converting enzyme inhibitor, calcium antagonist, fixed‐dose combination, hypertension
1. INTRODUCTION
Hypertension affects approximately 24.1% of men and 20.1% of women, corresponding to a total of 1.13 billion adults worldwide.1 Many different antihypertensive drug combinations have been shown to be effective against this condition2, 3, 4, 5, 6, 7; however, poor medication persistence and adherence are common problems in hypertensive patients, partly because of complex treatment regimens and high pill burdens.8, 9 This results in sustained periods of uncontrolled hypertension, leading to disease progression and associated economic consequences.9
One approach to maximizing adherence is the use of a fixed‐dose combination (FDC), whereby 2 or more antihypertensive agents are prescribed in a single pill. Compared to multipill free‐dose combinations, this simplifies drug‐taking while maintaining a comparable reduction in blood pressure.10 A major shortcoming of FDCs is their lack of flexibility, with physicians limited to the agent and dose combinations available on the market.
There is currently a lack of data regarding the differences in patient characteristics and comedications between individuals prescribed an FDC and those prescribed a free‐dose combination. Furthermore, the potential motivations behind prescription of one or another of the combination types have not been formally distinguished. The aim of the present analysis was to provide concrete data for both of these knowledge gaps, as well as to add to the growing pool of information regarding the effect of FDCs on persistence, adherence, and medication costs.
2. METHODS
Data from the IMS® Disease Analyzer (QuintilesIMS) were used for the present analysis. This data collection system contains electronic medical records for over 20 million patients, provided by 2500 physician practices across Germany. Data are fully anonymized and their use is in compliance with section 3, paragraph 6 of the Federal Data Protection Act (BDSG), 2009.
2.1. Patients
For the present analysis, only medical records supplied by general practitioners/diabetologists and pertaining to hypertensive patients who filled at least 1 prescription for concomitant R/A or C/A, either as a single‐pill FDC or as a 2‐pill free‐dose combination, were considered. Use of additional medications was permitted. Monthly data collection took place between August 2012 and February 2017.
2.2. Data management and definitions
The extracted data included details of patient demographics, medication, and clinical characteristics. For each patient, baseline was considered the point at which the first prescription for 1 of the 2 relevant combinations was filled. The number and class of antihypertensive therapies used immediately before and after this time were documented. Time to termination of combination therapy was also recorded, as was the time elapsed between filling of separate prescriptions. Patients were considered to be persisting with their antihypertension therapy if they were recorded to have filled their most recent prescription at each 30‐day interval after therapy initiation. Adherence is presented in the form of medication possession ratio (MPR), calculated by dividing the expected number of days covered by a prescription by the number of days that actually elapsed before the next prescription was filled.11, 12 Only patients with at least 2 prescriptions for the FDC medication or 2 for each of the agents in the free combination were included in this analysis. To ensure that findings reflected adherence rather than discontinuation, all data relating to the latest prescription time period (ie, the period that should have been covered by the last prescription filled by a patient) were discounted. Patients were then classified as having very good adherence (MPR > 0.90), good adherence (MPR 0.80‐0.90), poor adherence (MPR 0.50‐0.79), or very poor adherence (MPR < 0.50). The cost per patient year (PPY) of each FDC and free‐dose counterpart was calculated based on the pharmacy sales prices for the years in question.
The following comorbidities were recorded: hyperlipidemia, diabetes mellitus, congestive heart disease (CHD), angina pectoris, PAD, atherosclerosis, heart failure, cardiac arrhythmia, myocardial infarction (MI), history of stroke, liver disease, kidney disease, stomach disease, and dementia.
2.3. Statistical analysis
Data are presented as means ± standard deviation (SD) or absolute numbers and percentages. Differences between fixed and free combination groups were assessed using a chi‐square or Wilcoxon signed‐rank test, with an alpha level of 5%. In order to identify factors associated with the prescription of an FDC rather than a free‐dose combination, a multivariate logistic regression model was used, with adjustment for age, sex, region (Western vs Eastern Germany), number of antihypertensive therapy classes, and comorbidity. The results of this analysis are presented as odds ratios (OR) with 95% confidence intervals (CI) and P values. Time to therapy discontinuation was evaluated using Kaplan–Meier analysis, with patients censored if no information regarding the study medication was available at the 30‐day data submission point. Multivariate Cox regression analysis was used to compare the risk of therapy discontinuation between the fixed and free combinations. These data were adjusted for potential confounders, including age, sex, region (Western vs Eastern Germany), number of antihypertensive therapy classes, and comorbidity. All statistical analysis was carried out using SAS version 9.3 (Cary, NC, USA).
3. RESULTS
A total of 81 958 patients met criteria for inclusion in the study. Of the 71 463 (87.2%) who were prescribed R/A, 10 938 (15.3%) were receiving an FDC and 60 525 (84.7%) a free‐dose combination. Of the 10 495 patients who were prescribed C/A, 1413 (13.5%) were receiving an FDC and 9082 (86.5%) a free‐dose combination.
3.1. Patient characteristics
Patients receiving an FDC of R/A were younger (63.4 ± 14.1 vs 68.9 ± 14.1 years; P < .001) and more commonly male (56.2% vs 49.9%; P < .0001) than those receiving a free‐dose combination of the same antihypertensive agents (Table 1), with the same trend apparent for patients receiving a C/A combination (65.5 ± 13.1 vs 70.0 ± 13.3 years; P < .001 and 49.5% vs 43.8%; P < .0001, respectively). Comorbidities were generally less common in patients on an FDC. However, hyperlipidemia, liver disease, and stomach disease were all slightly more common in those on a fixed‐dose compared to a free‐dose R/A combination (40.1% vs 38.5%, P = .001; 14.2% vs 12.0%, P < .001; and 30.1% vs 29.2%, P = .046, respectively), with no significant differences between groups for patients taking C/A.
Table 1.
Baseline patient characteristics
| Ramipril/amlodipine combination | Candesartan/amlodipine combination | |||||
|---|---|---|---|---|---|---|
| Fixed‐dose N = 10 938 | Free‐dose N = 60 525 | P value | Fixed‐dose N = 1413 | Free‐dose N = 9082 | P value | |
| Age (y) | 63.4 ± 14.1 | 68.9 ± 14.1 | < .001 | 64.5 ± 13.1 | 70.0 ± 13.3 | < .001 |
| Male sex | 56.2 | 49.9 | < .0001 | 49.5 | 43.8 | < .0001 |
| Private insurance | 4.9 | 5.3 | .135 | 9.1 | 10.0 | .303 |
| Comorbidities | ||||||
| Hyperlipidemia | 40.1 | 38.5 | .001 | 42.9 | 44.1 | .402 |
| Diabetes mellitus | 28.0 | 31.5 | < .001 | 28.8 | 31.2 | .071 |
| CHD | 14.5 | 23.1 | < .001 | 14.4 | 25.2 | <.001 |
| Angina pectoris | 4.9 | 5.1 | .209 | 5.7 | 6.6 | .165 |
| PAD | 6.3 | 8.0 | < .001 | 6.3 | 8.5 | .005 |
| Atherosclerosis | 4.8 | 5.3 | .016 | 5.6 | 6.9 | .061 |
| Heart failure | 7.9 | 12.2 | < .001 | 7.7 | 12.8 | < .001 |
| Cardiac arrhythmia | 11.2 | 16.7 | < .001 | 13.1 | 19.9 | < .001 |
| MI | 2.0 | 4.1 | < .001 | 1.6 | 3.9 | < .001 |
| History of stroke | 5.9 | 10.2 | < .001 | 5.6 | 10.0 | < .001 |
| Liver disease | 14.2 | 12.0 | < .001 | 15.6 | 14.6 | .213 |
| Kidney disease | 7.7 | 11.1 | < .001 | 10.2 | 13.9 | < .001 |
| Stomach disease | 30.1 | 29.2 | .046 | 35.2 | 36.1 | .503 |
| Dementia | 3.3 | 6.4 | < .001 | 2.6 | 5.3 | < .001 |
| No. of comorbidities | ||||||
| 0 | 27.0 | 22.8 | < .0001 | 24.5 | 18.9 | < .0001 |
| 1 | 26.0 | 22.7 | 25.2 | 20.9 | ||
| 2 | 19.3 | 19.0 | 18.8 | 20.0 | ||
| 3 | 12.0 | 14.0 | 13.4 | 15.1 | ||
| 4 | 7.2 | 9.3 | 8.1 | 9.7 | ||
| 5 | 4.1 | 5.8 | 4.5 | 6.7 | ||
| ≥ 6 | 4.4 | 6.5 | 5.4 | 8.6 | ||
| Blood pressurea | ||||||
| SBP | 153.5 ± 23.4 | 150.5 ± 24.2 | < .001 | 152.1 ± 22.3 | 149.0 ± 24.4 | .123 |
| DBP | 87.6 ± 12.8 | 85.5 ± 13.9 | < .001 | 85.5 ± 13.5 | 83.5 ± 13.5 | .013 |
| No. of antihypertensive agents in use prior to baseline | 2.7 ± 2.0 | 2.9 ± 2.2 | < .001 | 2.9 ± 2.0 | 3.4 ± 2.3 | < .001 |
| 0 agents | 11.9 | 14.5 | < .001 | 7.4 | 8.3 | .235 |
| 1 agent | 16.0 | 12.2 | < .001 | 13.8 | 8.9 | < .001 |
| 2 agents | 23.8 | 19.6 | < .001 | 23.1 | 18.4 | < .001 |
| ≥ 3 agents | 48.3 | 53.7 | < .001 | 55.7 | 64.5 | < .001 |
CHD, coronary heart disease; DBP, diastolic blood pressure; MI, myocardial infarction; PAD, peripheral artery disease; SBP, systolic blood pressure.
n‐numbers as follows: fixed‐dose R/A: 2158; free‐dose R/A: 8653; fixed‐dose C/A: 198; free‐dose C/A: 1235.
For the patients on R/A, those prescribed an FDC had been taking, on average, a slightly lower number of antihypertensive medications prior to baseline compared to those prescribed a free‐dose combination (2.7 ± 2.0 vs 2.9 ± 2.2; P < .001) (Table 1). The same was true for patients on C/A (2.9 ± 2.0 vs 3.4 ± 2.3; P < .001). In both R/A and C/A patients, a significantly higher proportion of the free‐dose compared to FDC group had been receiving ≥ 3 antihypertensive agents prior to baseline (53.7% vs 48.3%; P < .001 and 64.5% vs 55.7%; P < .001, respectively).
3.2. Baseline predictors of combination type
For both R/A and C/A patients, having hyperlipidemia was associated with a greater likelihood of being prescribed an FDC rather than a free‐dose combination (OR: 1.26, 95% CI, 1.21‐1.32; P < .001 and OR: 1.19, 95% CI, 1.05‐1.34; P = .007) (Figure 1A,B). Conversely, prior MI (OR: 0.61, 95% CI, 0.52‐0.60; P < .001 and OR: 0.60, 95% CI, 0.38‐0.95; P = .027), prior stroke (OR: 0.68, 95% CI, 0.62‐0.74; P < .001 and OR: 0.70, 95% CI, 0.55‐0.89; P = .004), and coronary heart disease (CHD; OR: 0.68, 95% CI, 0.64‐0.73; P < .001 and OR: 0.64, 95% CI, 0.54‐0.77; P < .001) were found to be negative predictors of treatment with an FDC. In R/A patients only, liver disease, angina pectoris, stomach disease, and male sex were also identified as positive predictors of FDC use, whereas dementia, cardiac arrhythmia, kidney failure, and heart failure were further negative predictors. None of these was significantly associated with FDC prescription in C/A patients. There was no effect of the number of antihypertensive agents in use prior to baseline (OR 1.0; 95% CI, 0.99‐1.01; data not shown).
Figure 1.
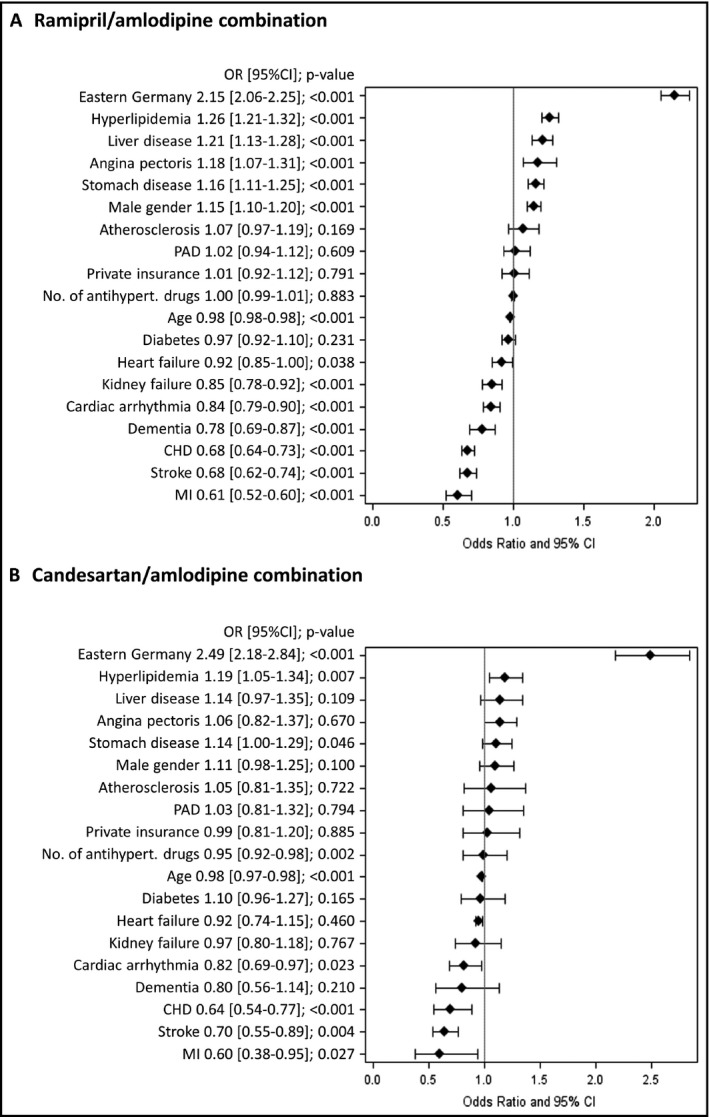
Factors associated with patients prescribed a fixed‐dose vs free‐dose combination. CHD, congestive heart failure; MI, myocardial infarction; PAD, peripheral artery disease
3.3. Antihypertensive comedication
Compared to patients on a free‐dose combination, those on an FDC were less likely to be taking any additional antihypertensive comedication during the study (R/A: OR: 0.78, 95% CI, 0.72‐0.8; P < .001; C/A: OR: 0.55, 95% CI, 0.48‐0.61, P < .001) (Table 2). Overall, beta‐blockers and diuretics were the most commonly prescribed additional agents.
Table 2.
Use of antihypertensive comedications in patients on fixed‐dose vs free‐dose combinations
| Ramipril/amlodipine combination | Candesartan/amlodipine combination | |||||||
|---|---|---|---|---|---|---|---|---|
| Fixed‐dose N = 10 938 | Free‐dose N = 60 525 | Adj. OR (95% CI)a | P value | Fixed‐dose N = 1413 | Free‐dose N = 9082 | Adj. OR (95% CI)a | P value | |
| Any | 53.1 | 69.5 | 0.78 (0.72‐0.84) | < .001 | 44.9 | 64.8 | 0.55 (0.48‐0.61) | < .001 |
| Diuretic | 21.0 | 36.2 | 0.58 (0.56‐0.60) | < .001 | 18.7 | 35.3 | 0.54 (0.46‐0.62) | < .001 |
| β‐blocker | 36.9 | 48.1 | 0.73 (0.70‐0.76) | < .001 | 31.1 | 44.4 | 0.67 (0.59‐0.76) | < .001 |
| CA | 3.2 | 4.0 | 0.88 (0.78‐0.98) | .025 | 3.6 | 5.3 | 0.76 (0.56‐1.03) | .072 |
| ACE‐i | 6.2 | 13.3 | 0.42 (0.39‐0.46) | < .001 | 3.3 | 4.1 | 0.89 (0.64‐1.22) | .456 |
| ARB | 7.2 | 7.0 | 0.99 (0.91‐1.07) | .786 | 6.7 | 10.3 | 0.62 (0.50‐0.78) | < .001 |
ACE‐i, angiotensin‐converting enzyme inhibitor; Adj., adjusted; ARB, angiotensin receptor blocker; CA, calcium antagonist.
Adjusted for age, sex, region, and comorbidity.
3.4. Blood pressure values
Overall, both systolic (SBP) and diastolic (DBP) blood pressure values fell between baseline and follow‐up; however, the magnitude of this change was not significantly different between patients receiving an FDC and those receiving a fixed‐dose combination (Figure 2A,B). This was true for patients on a R/A combination (SBP: −8.7 vs −9.1 mm Hg; DBP: −4.1 vs 4.1 mm Hg, respectively) and those on a C/A combination (SBP: −8.4 and −7.5 mm Hg; DBP: −3.7 and −3.5 mm Hg, respectively).
Figure 2.
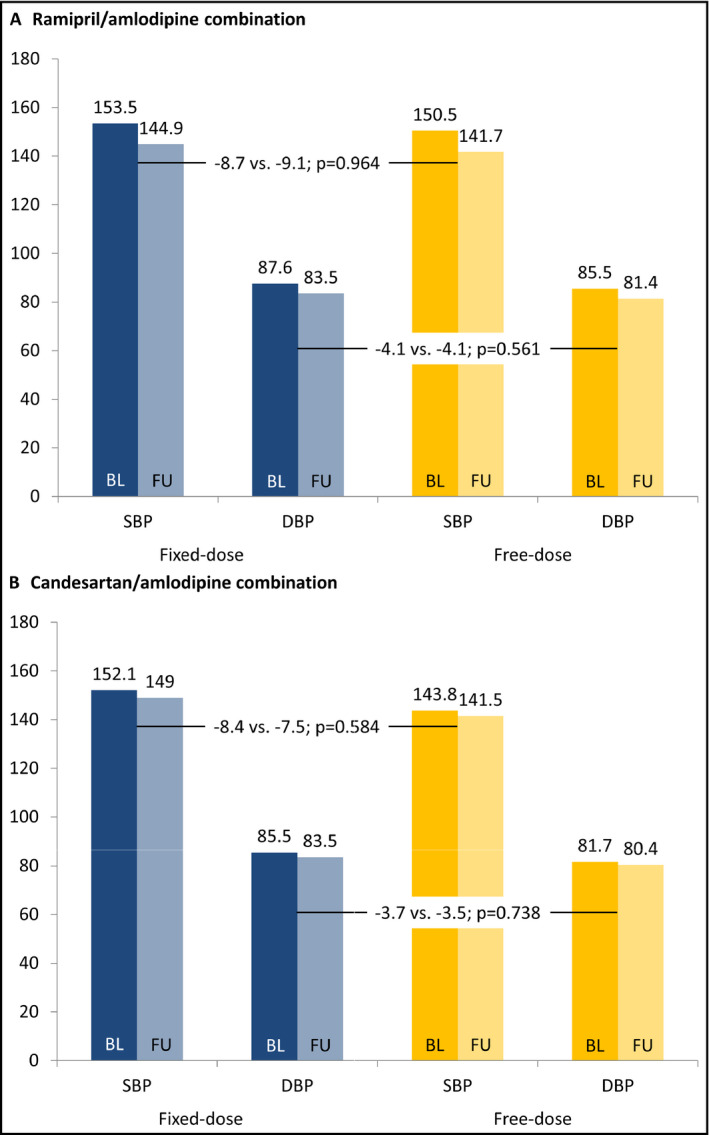
Change in blood pressure between baseline and follow‐up. P values adjusted for age, sex, region, cotherapy and comorbidity at baseline. Follow‐up took place between 30 and 365 d post enrollment
3.5. Persistence/adherence
Up to 2 months post initiation, comparable proportions of patients on an FDC and free‐dose combination stopped taking the study medication. However, rates of treatment cessation were higher in free‐dose combination patients from 3 months onwards, with the likelihood of discontinuation by 12 months significantly lower in patients taking an FDC (R/A: 65.7% vs 48.6%, HR: 0.65, 95% CI, 0.58‐0.73; P < .001; C/A: 55.5% vs 43.1%, HR: 0.82, 95% CI, 0.80‐0.84; P < .001) (Figure 3A,B).
Figure 3.
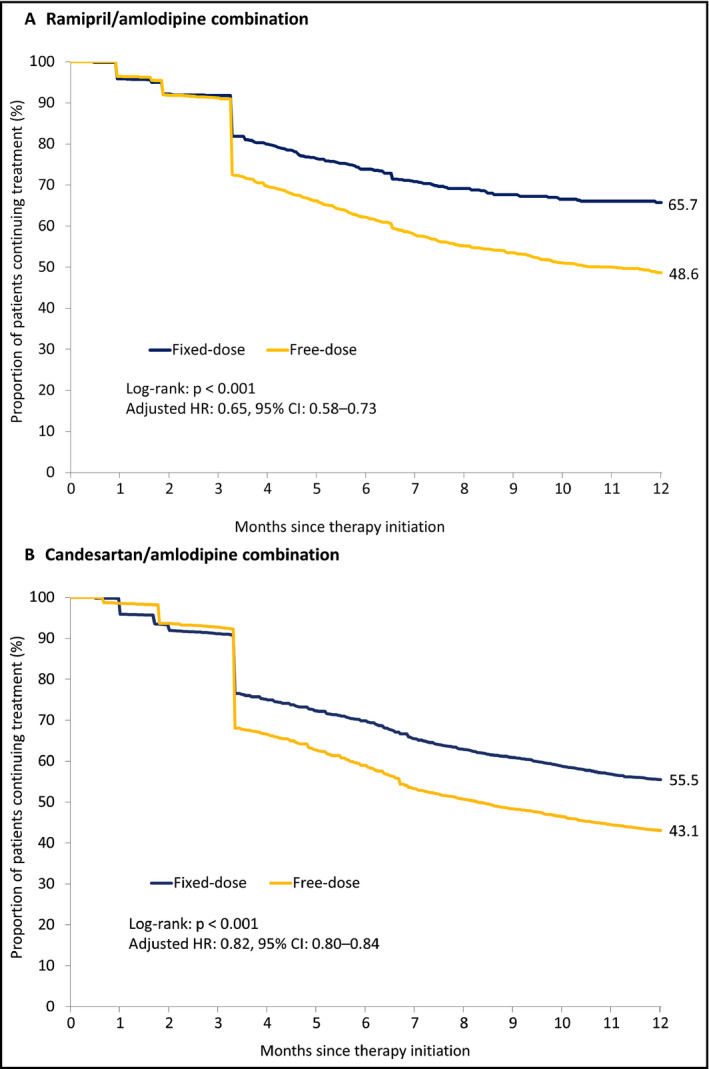
Persistence over the 12 mo following therapy initiation. Hazard ratios adjusted for age, sex, region, cotherapy and comorbidity at baseline
Higher proportions of patients on an FDC demonstrated very good or good adherence to combination treatment during the initial 12 months compared to their free‐dose combination counterparts (R/A: 37.7% vs 20.0% and 14.4% vs 12.9%; P < .001 overall; C/A: 62.9% vs 40.1% and 21.7% vs 18.1%; P < .001 overall) (Figure 4A,B). The mean MPR was higher for patients taking an FDC compared to those taking a free‐dose combination (R/A: 0.72 vs 0.58; P < .001; C/A: 0.92 vs 0.79; P < .001).
Figure 4.
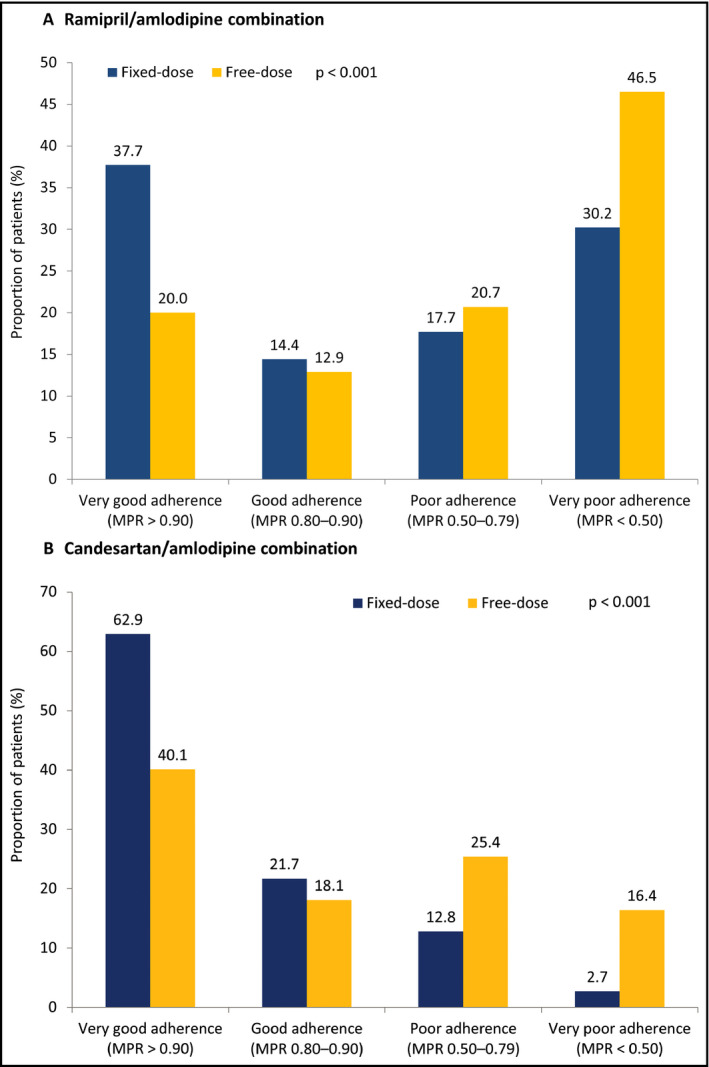
Adherence during the first 12 mo after therapy initiation. MPR, medication possession ratio. For (A) and (B), P < .001 for the distribution of fixed‐ vs free‐dose combination patients across adherence subcategories
3.6. Costs of combination therapy
The cost PPY of treatment with an FDC was significantly higher than that of treatment with a free‐dose combination (R/A: €230.20 vs €134.16; P < .001; C/A: €339.61 vs €235.01; P < .001) (Figure 5). After adjustment for age, sex, and comorbidities, the corresponding cost difference was +€98.90 PPY for the R/A FDC and €107.71 PPY for the C/A FDC.
Figure 5.
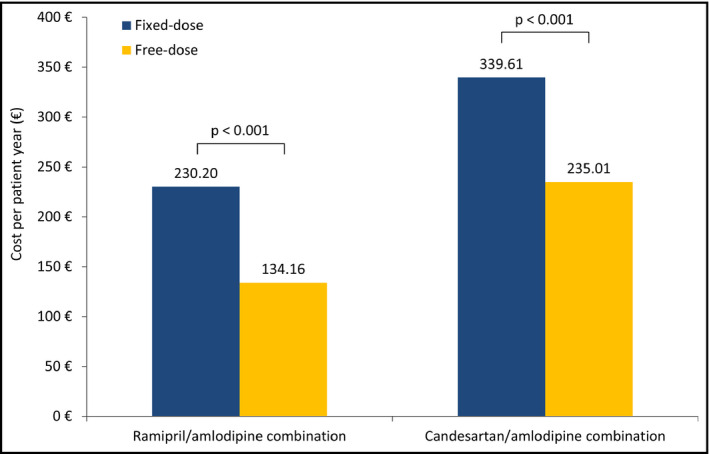
Cost of fixed‐dose compared to free‐dose combination per patient per year
4. DISCUSSION
Compared to patients being initiated on a free‐dose combination, those being initiated on an FDC were generally younger and less comorbid. Indeed, at multivariate analysis, prior MI, prior stroke, and CHD were shown to be negative predictors of FDC prescription, suggesting that physicians prefer more flexible treatment in patients with these characteristics. Patients managed with an FDC were less likely to be prescribed any additional antihypertensive comedication and more likely to be persisting with their treatment at 12 months. Adherence was also found to be superior in FDC compared to free‐dose combination groups, supporting previous findings.
4.1. Factors associated with the prescription of an FDC rather than a free‐dose combination
Patients being initiated on an FDC were approximately 5 years younger and less comorbid than those being initiated on a free‐dose combination. These 2 characteristics are likely closely related, with the prevalence of conditions such as CHD, stroke, and MI known to increase with advancing age.13, 14, 15, 16 Indeed, at adjusted multivariate analysis, these particular comorbidities were found to be significant negative predictors of treatment with an FDC, whereas no association was seen for age. This suggests that concomitant cardiovascular conditions are key drivers for use of a free‐dose combination rather than an FDC.
A 20/10 mm Hg rise in blood pressure has been shown to result in approximately a 2‐fold increase in the risk of fatal ischemic heart disease or stroke in patients aged between 40 and 90 years.17 Consequently, aggressive antihypertensive therapy is particularly necessary in patients with existing CHD or a history of cardiovascular events, who are already at high risk of recurrence. The facility to freely modify individual antihypertensive agent doses is considered advantageous under such circumstances, likely explaining the preference for free‐dose combinations in this patient population. However, patients with more complex disease also tend to be prescribed a greater number of drugs. In this case, use of an FDC would reduce the pill burden, encouraging treatment adherence, which may translate into better outcomes.8, 9 In future, the availability of a wider range of FDCs with more dose and agent combinations will, it is hoped, allow a greater spectrum of patients to benefit.
Despite their lower age, patients on an FDC had a similar frequency of angina pectoris, liver disease, and stomach disease compared to those on a free‐dose combination. Although these were found to be statistically significant predictors of management with a C/A FDC at multivariate analysis, their mild effect and lack of significance for R/A combinations suggests that they are not particularly decisive influences when deciding between combination type. However, for both drug pairings evaluated herein, hyperlipidemia appeared to be associated with an increased likelihood of an FDC being prescribed, rather than a free‐dose combination. The reason for this is not immediately clear and merits further investigation.
A further consideration is the number of antihypertensive agents that are being taken by a patient before starting the present combination therapy. Our data suggest that between 19% and 27% of patients who received a free‐dose combination were already being treated with 3 or more drugs prior to baseline, compared to just 15%‐18% of those who received an FDC. This suggests a preference for the addition of a free agent where a patient's pill burden is already high. Although this practice may facilitate greater flexibility in terms of dosing, all current evidence suggests that adherence would suffer as a consequence.8, 9 Instead, such patients may benefit from substitution of an existing medication with an FDC for further treatment intensification. Studies that explore this theory would be informative.
Besides clinical variables, the potential influence of physician preference and degree of familiarity with/attitude toward ESC guidelines, which promote use of FDCs over free‐dose combinations, cannot be overlooked.7 For example, previous studies have associated older practitioner age with poorer adherence to guidelines on the management of hypertension.18 Furthermore, the disparity between prescription behaviors in what were formerly Eastern and Western Germany may also be related to physician factors. Evidence suggests that in the east, the average age of practitioners is lower and hypertension is more prevalent19, 20; thus, it may be speculated that doctors in the east refer and adhere to guidelines more often. Unfortunately, the current data do not permit exploration into such theories.
4.2. Additional antihypertensive co‐medications whilst on combination therapy
FDC patients were significantly less likely than free‐dose combination patients to receive any additional antihypertensive cotherapy. Addition of a third antihypertensive agent is indicated when satisfactory blood pressure control is not achieved on optimized dual‐agent therapy,7 which suggests that a higher proportion of the FDC patients had attained adequate control using the dual combination alone.
A known source of pseudoresistant hypertension is poor adherence to antihypertensive medication. Physicians may often prescribe additional agents when faced with high blood pressure readings, when stricter observance of existing regimens may be more appropriate.21, 22 The poorer adherence observed in the free‐dose combination groups in the present study could be a possible explanation for the higher prevalence of additional antihypertensive cotherapy use in this patient subset.
4.3. Persistence and adherence
A higher proportion of patients receiving an FDC were still taking their medication at 12 months compared to those on a free‐dose combination, with their odds of treatment persistence at this time point found to be 16%‐42% greater. This may be related to the higher number of agents being used by fixed‐dose patients, meaning that regimens were more likely to be subject to modifications. Similarly, good or very good adherence was seen in approximately 20%‐25% more patients on an FDC compared to those on a free‐dose combination. These figures are in line with findings from previous studies, with a large‐scale meta‐analysis reporting approximately a 54% higher likelihood of persistence and a 21% higher likelihood of adherence in patients on an FDC compared to a free‐dose combination.10 Further support for the ability of FDCs to improve adherence comes from a study in which patients were prescribed 3 antihypertensive agents in the form of 1, 2, or 3 pills.23 The proportion of patients with adherence above 80% was found to fall with increasing pill burden (55.3%, 40.4%, and 32.6%, respectively). Thus, the evidence for this particular advantage of FDCs is compelling.
There is a large pool of evidence suggesting that improved adherence translates into superior outcomes. Of 3411 hypertensive patients in Poland who were switched from a free‐dose bisoprolol/amlodipine combination to a similar dose of the same agents on an FDC regimen, 97% had good or very good adherence at 6 months, with a mean fall in SBP and DBP of 16.3 and 8.8 mm Hg, respectively.24 Authors cite the excellent adherence as being the likely basis for these improvements. In addition, a recent analysis of data for 5680 hypertensive patients from the National Health Insurance Research Database (NHIRD) of Taiwan found those taking a fixed‐dose calcium antagonist/angiotensin receptor blocker (CA/ARB) combination to have superior adherence and persistence compared to those on a free‐dose CA/ARB combination, with the likelihood of experiencing a major adverse cardiac event (MACE) or hospitalization due to heart failure reduced by approximately 28% and 29%, respectively.25 Other studies have also demonstrated fewer cardiovascular events; fewer referrals to general, cardiology, or hypertension outpatient departments26; fewer visits to the emergency department; and fewer hospitalizations27, 28 in patients treated with FDCs compared to free‐dose combinations. Many authors cite improved adherence as the reason for these superior outcomes. Given that we were not able to provide data for these effects in the present analysis because of restrictions of the study design, future studies demonstrating these benefits specifically for R/A and C/A combinations would be useful.
Although not verified statistically, a nominally higher rate of persistence was seen for patients on aN R/A compared to a C/A combination, with adherence appearing to be better in those treated with the latter. Direct comparisons of this nature have not yet been made in the literature and may merit investigation in a well‐designed head‐to‐head study.
4.4. Treatment costs
As expected, fixed‐dose combinations of both R/A and C/A were more expensive per patient year than their free‐dose counterparts. This is largely owing to the wide availability of generic single‐component drugs in Germany, which can be more cheaply prescribed as multi‐pill regimens.29 However, this is an extremely minimalist view of expenditures in hypertensive patients, which additionally include the cost of other medications, outpatient care, emergency department visits, and hospital admissions.30 Indeed, the increased adherence associated with FDCs has been shown to translate into reduced health care use, which in turn results in cost savings that offset initial drug expenditure.26, 27, 28, 31, 32 Thus, the higher price of FDCs should not be a barrier to their use.
4.5. Limitations
First, although the large sample size is a strength of the present analysis, it may also have resulted in the statistical detection of small differences between FDC and free‐dose combination groups that were not clinically relevant. This is particularly pertinent for the R/A combination, for which there were an extremely high number of patients. Data should be interpreted with this in mind. Second, all data were obtained from a retrospective database updated by individual physicians unaware of the study. As a result, the accuracy of data is not always certain. Completeness of data is also limited by design, with treatment doses and outcomes over comparable timescales unobtainable, and certain influential variables such as exercise, nutritional behavior, and smoking status unaccounted for. Furthermore, several assumptions were made that may limit validity, such as that repeat prescription filling indicates that the previous pill supply has been exhausted, ergo the patient is adherent. Nevertheless, our findings are in line with the majority of published studies. Finally, the data presented here may not be generalizable to other drug combinations or to countries outside of Germany. The latter is particularly pertinent when considering treatment costs.
5. CONCLUSIONS
Patient comorbidity, particularly when of a cardiovascular nature, appears to be a deciding factor when choosing between an FDC and free‐dose combination antihypertensive therapy regime. This may reflect the need for more flexibility in higher‐risk patients, who are likely to require more aggressive therapy and more precisely titrated doses. Consequently, the limited choice of agents and doses in FDC pills may act as a deterrent for their use in such individuals. However, increased persistence and adherence were once again observed in patients on an FDC, which have been associated with better cardiovascular and economic outcomes, despite the higher initial cost of treatment. The emergence of a wider variety of FDCs, both in terms of dosages and antihypertensive agents, will facilitate greater flexibility and potentially allow patients with more complex disease to access these benefits. Similar analyses of different agent combinations are now merited.
CONFLICT OF INTEREST
Peter Bramlage received honoraria from Hexal AG, Holzkirchen for hypertension‐related medical advice. Stefanie Schmidt is an employee of Hexal. Helen Sims has no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
The database analysis was performed upon request of Hexal AG, Holzkirchen. Results were explored by all authors and Helen Sims provided a first draft that was critically revised by Peter Bramlage and Stefanie Schmidt. All authors approved the final manuscript version submitted and take full responsibility for the content of the manuscript.
ACKNOWLEDGMENTS
We acknowledge the expert statistical advice of Prof. Karel Kostev, IMS Frankfurt.
Bramlage P, Schmidt S, Sims H. Fixed‐dose vs free‐dose combinations for the management of hypertension—An analysis of 81 958 patients. J Clin Hypertens. 2018;20:705–715. 10.1111/jch.13240
REFERENCES
- 1. NCD‐RisC . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population‐based measurement studies with 19.1 million participants. Lancet. 2017;389:37‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hostalek U, Czarnecka D, Koch EMW. Treatment of hypertensive patients with a fixed‐dose combination of bisoprolol and amlodipine: results of a cohort study with more than 10,000 patients. Cardiol Ther. 2015;4:179‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laurent S, Parati G, Chazova I, et al. Randomized evaluation of a novel, fixed‐dose combination of perindopril 3.5 mg/amlodipine 2.5 mg as a first‐step treatment in hypertension. J Hypertens. 2015;33:653‐661; discussion 662. [DOI] [PubMed] [Google Scholar]
- 4. Takase B, Nagata M. Fixed‐dose combination of losartan and hydrochlorothiazide significantly improves endothelial function in uncontrolled hypertension by low‐dose amlodipine: a randomized study. Anadolu Kardiyol Derg. 2014;14:685‐691. [DOI] [PubMed] [Google Scholar]
- 5. Rakugi H, Tsuchihashi T, Shimada K, et al. Efficacy and safety of fixed‐dose losartan/hydrochlorothiazide/amlodipine combination versus losartan/hydrochlorothiazide combination in Japanese patients with essential hypertension. Clin Exp Hypertens. 2015;37:260‐266. [DOI] [PubMed] [Google Scholar]
- 6. Chrysant SG. Effectiveness of the fixed‐dose combination of olmesartan/amlodipine/hydrochlorothiazide for the treatment of hypertension in patients stratified by age, race and diabetes, CKD and chronic CVD. Expert Rev Cardiovasc Ther. 2013;11:1115‐1124. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281‐1357. [DOI] [PubMed] [Google Scholar]
- 8. Gupta P, Patel P, Štrauch B, et al. Risk factors for nonadherence to antihypertensive treatment. Hypertension. 2017;69:1113‐1120. [DOI] [PubMed] [Google Scholar]
- 9. Vrijens B, Antoniou S, Burnier M, de la Sierra A, Volpe M. Current situation of medication adherence in hypertension. Front Pharmacol. 2017;8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed‐dose combinations of antihypertensive agents: a meta‐analysis. Hypertension. 2010;55:399‐407. [DOI] [PubMed] [Google Scholar]
- 11. Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry‐Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3‐12. [DOI] [PubMed] [Google Scholar]
- 12. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280‐1288. [DOI] [PubMed] [Google Scholar]
- 13. Davis JW, Chung R, Juarez DT. Prevalence of comorbid conditions with aging among patients with diabetes and cardiovascular disease. Hawaii Med J. 2011;70:209‐213. [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly‐Hayes M. Influence of age and health behaviors on stroke risk: lessons from longitudinal studies. J Am Geriatr Soc. 2010;58:S325‐S328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29‐e322. [DOI] [PubMed] [Google Scholar]
- 16. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903‐1913. [DOI] [PubMed] [Google Scholar]
- 18. Adedeji AR, Tumbo J, Govender I. Adherence of doctors to a clinical guideline for hypertension in Bojanala district, North‐West Province, South Africa. Afr J Prim Health Care Fam Med. 2015;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helmert U, Mielck A, Classen E. Social inequities in cardiovascular disease risk factors in East and West Germany. Soc Sci Med. 1992;35:1283‐1292. [DOI] [PubMed] [Google Scholar]
- 20. Luschen G, Niemann S, Apelt P. The integration of two health systems: social stratification, work and health in East and West Germany. Soc Sci Med. 1997;44:883‐899. [DOI] [PubMed] [Google Scholar]
- 21. Weber F, Anlauf M. Treatment resistant hypertension – investigation and conservative management. Dtsch Arztebl Int. 2014;111:425‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31:766‐774. [DOI] [PubMed] [Google Scholar]
- 23. Xie L, Frech‐Tamas F, Marrett E, Baser O. A medication adherence and persistence comparison of hypertensive patients treated with single‐, double‐ and triple‐pill combination therapy. Curr Med Res Opin. 2014;30:2415‐2422. [DOI] [PubMed] [Google Scholar]
- 24. Czarnecka D, Koch EM, Gottwald‐Hostalek U. Benefits of a fixed‐dose combination of bisoprolol and amlodipine in the treatment of hypertension in daily practice: results of more than 4000 patients. Curr Med Res Opin. 2015;31:875‐881. [DOI] [PubMed] [Google Scholar]
- 25. Tung YC, Huang YC, Wu LS, Chang CJ, Chu PH. Medication compliance and clinical outcomes of fixed‐dose combinations vs free combinations of an angiotensin II receptor blocker and a calcium channel blocker in hypertension treatment. J Clin Hypertens (Greenwich). 2017;19:983‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belsey JD. Optimizing adherence in hypertension: a comparison of outcomes and costs using single tablet regimens vs individual component regimens. J Med Econ. 2012;15:897‐905. [DOI] [PubMed] [Google Scholar]
- 27. Hess G, Hill J, Lau H, Dastani H, Chaudhari P. Medication utilization patterns and hypertension‐related expenditures among patients who were switched from fixed‐dose to free‐combination antihypertensive therapy. P T. 2008;33:652‐666. [PMC free article] [PubMed] [Google Scholar]
- 28. Yang W, Chang J, Kahler KH, et al. Evaluation of compliance and health care utilization in patients treated with single pill vs. free combination antihypertensives. Curr Med Res Opin. 2010;26:2065‐2076. [DOI] [PubMed] [Google Scholar]
- 29. Hong SH, Wang J, Tang J. Dynamic view on affordability of fixed‐dose combination antihypertensive drug therapy. Am J Hypertens. 2013;26:879‐887. [DOI] [PubMed] [Google Scholar]
- 30. Hilleman DE. Adherence and health care costs with single‐pill fixed‐dose combinations in hypertension management. J Manag Care Pharm. 2014;20:93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single‐pill vs free‐equivalent combination therapies for hypertension: a meta‐analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13:898‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tung Y‐C, Lin Y‐S, Wu L‐S, Chang C‐J, Chu P‐H. Clinical outcomes and healthcare costs in hypertensive patients treated with a fixed‐dose combination of amlodipine/valsartan. J Clin Hypertens (Greenwich). 2015;17:51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]


