Figure 1.
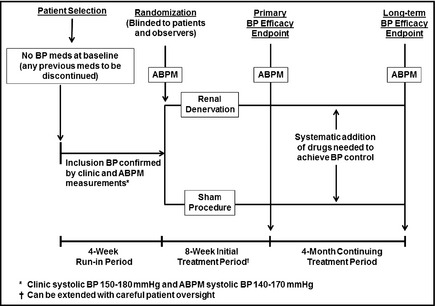
Renal Denervation Alone. This is a three‐period trial design. Period 1: Potentially eligible hypertensive patients are screened into a 4‐week run‐in period during which any previous blood pressure (BP) drugs are discontinued and stable BP is achieved. Patients meeting inclusion criteria, including clinic BP and ambulatory BP monitoring (ABPM) values, are randomized to active renal denervation or a sham procedure. Other than the intervention team, who has no patient contact after the procedure, all other staff members as well as patients are blinded to the assignment. Period 2: After an 8‐week initial treatment period, during which no BP medications can be added (except for emergencies), systolic BP by ABPM is used as the primary study endpoint; clinic BPs are a secondary endpoint. Period 3: Double‐blind status is maintained during a 4‐month period during which investigators systematically add medications or adjust doses according to a strict protocol, at 2‐ to 3‐week intervals, until BP control (clinic systolic BP <140 mm Hg) is achieved. ABPM and clinic BPs at the end of this period are secondary endpoints, as is the medication score (derived from the number of treatment steps at which drugs are added or doses increased). Safety endpoints: These include evidence for renal artery damage, decreases in renal function, or any other adverse findings possibly resulting from the procedure. Safety will continue to be monitored for up to 2 years following this trial.
