Figure 3.
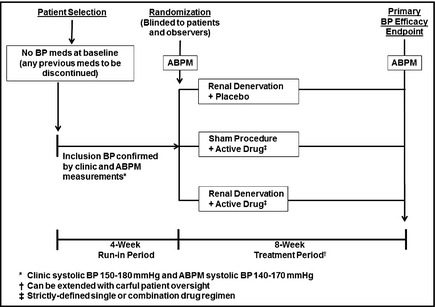
A Definitive Combination Therapy Protocol. This is a two‐part trial design. Period 1: Potentially eligible hypertensive patients are screened into a 4‐week run‐in period during which any previous blood pressure (BP) drugs are discontinued and stable BP is achieved; a single‐blind placebo could be administered during this run‐in period. Patients meeting inclusion criteria, including clinic BP and ambulatory BP monitoring (ABPM) values, are randomized to one of three treatment arms: active renal denervation alone (plus drug placebo), active drug therapy alone (plus sham denervation), or the combination of denervation plus active drug therapy. Other than the intervention team, who will have no patient contact after the procedure, all other staff members as well as patients are blinded to the assignment. Period 2: After an 8‐week treatment period, during which no BP medications can be added (except for emergencies), systolic BP by ABPM is used as the primary study endpoint; clinic BPs are a secondary endpoint. It is assumed that the active drug therapy in this trial will generally be a single agent. It would also be possible to use a two‐drug combination, but it would be recommended that the clinic systolic BP criterion for randomization be ≥160 mm Hg and the ABPM be ≥145 mm Hg to allow a BP range sufficiently high to adequately assess the additive effects of denervation when combined with such a drug regimen. Safety endpoints: These include evidence for renal artery damage, decreases in renal function, or any other adverse findings possibly resulting from the procedure. Safety will continue to be monitored for up to 2 years following this study.
