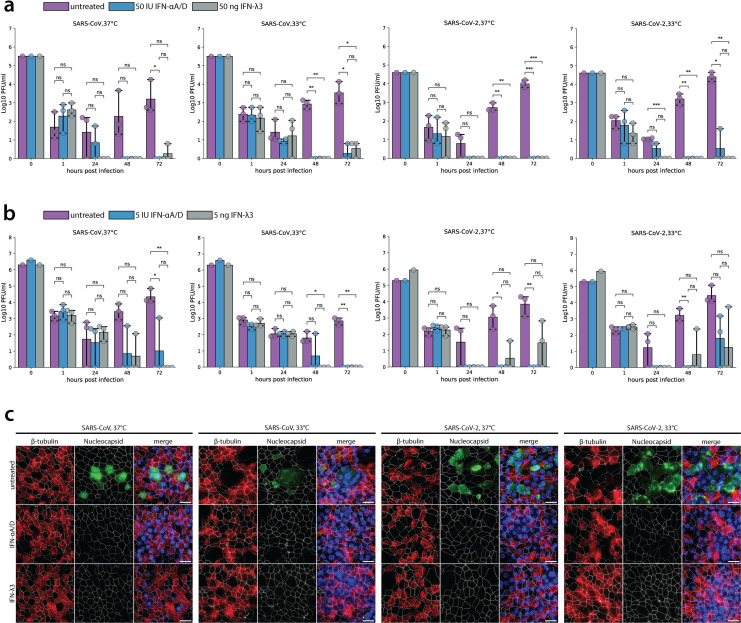
Fig 2. SARS-CoV and SARS-CoV replication upon IFN-I and IFN-III pretreatment.
hAEC cultures were treated from the basolateral side with recombinant universal type I IFN (50 IU or 5 IU) or recombinant IFN-λ3 (50 ng or 5 ng) for 18 hours. Before infection, medium was removed and replaced with IFN-free medium, and hAEC cultures were infected with SARS-CoV and SARS-CoV-2 using 30,000 PFU and were incubated at 37°C or 33°C. Inoculated virus was removed at 1 hpi, and the apical side was washed. Cultures were further incubated at the indicated temperature. At the indicated time, apical virus release was assessed by plaque titration (a, b). Data represent the mean ± 95% CI of hAEC cultures from 3 different human donors. Individual points represent 1 (b) or the average of 2 technical replicates (a). Values at 0 hpi indicate the titer of the inoculum used to infect the hAEC cultures, and values at 1 hpi indicate the remaining titer after the third wash. The p-values were computed by using two-sided paired sample t tests. The data underlying this figure are found in S1 Data. At 72 hpi, hAEC cultures pretreated with 50 IU type I IFN or 50 ng type III IFN were fixed and processed for immunofluorescence analysis using antibodies against SARS-CoV Nucleocapsid protein (green), β-tubulin (cilia, red), ZO-1 (tight junctions, white), and DAPI (blue) (c). Representative z-projections of 1 donor are shown. Scale bar, 20 μm. hAEC, human airway epithelial cell; hpi, hours post infection; IFN, interferon; PFU, plaque-forming unit; SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.