Figure 4.
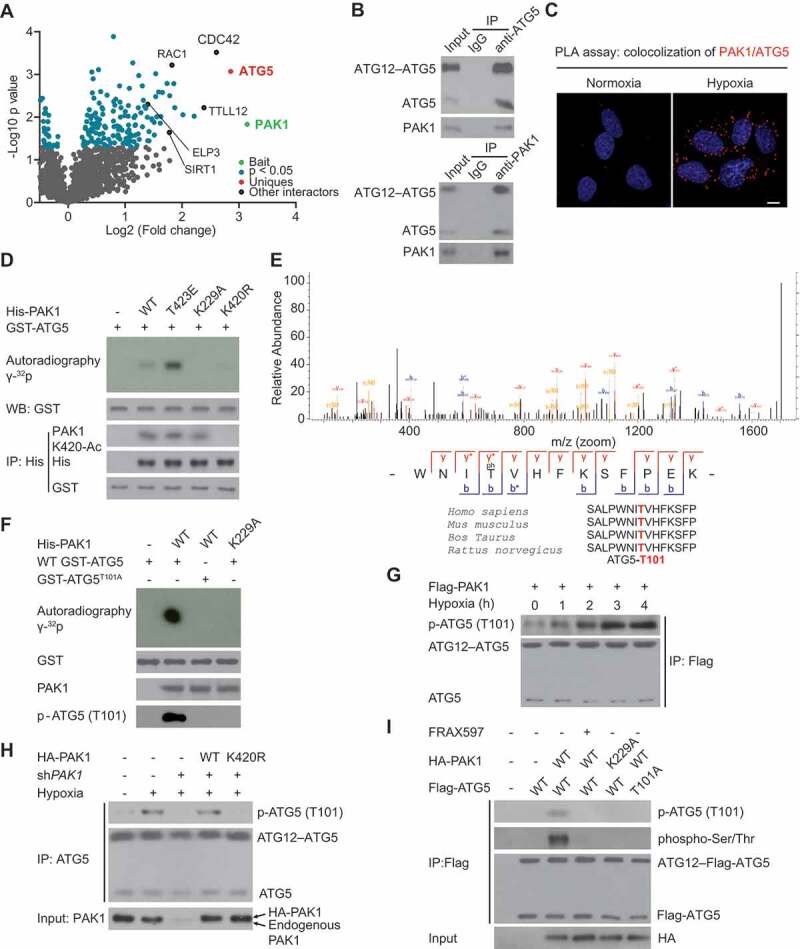
PAK1 directly phosphorylates ATG5 at conserved T101 residue in GBM cells. (A) Volcano plot indicating the interactors of PAK1 in U87 cells. (B) Hypoxia-induced the co-immunoprecipitation of endogenous PAK1 and ATG5 in LN229 cells. (C) PLA assay indicated that hypoxia promoted the cytoplasmic co-localization of endogenous PAK1 and ATG5 in LN229 cells. Scale bar: 0.01 mm. (D) Ni-NTA agarose beads were used to immobilize bacterially purified His-PAK1 proteins, then incubated with HA-ELP3 and Ac-CoA. Then these beads were incubated with purified GST-ATG5 and [γ-32P] ATP kinase buffer. Autoradiography was performed. (E) Mass spectrometric analysis was performed to identify the PAK1-induced phosphorylation site of ATG5. (F) Ni-NTA agarose beads were used to immobilize bacterially purified His-PAK1 proteins as indicated. Then these beads were incubated with GST-ATG5 or GST-ATG5T101A mutant and [γ-32P] ATP kinase buffer. ATG5 phosphorylation was examined. (G) LN229 cells with Flag-PAK1 transfection were submitted to hypoxia treatment or not. Immunoprecipitation (IP) with anti-Flag was performed. A specific anti-p-ATG5 (T101) antibody produced by our group was used to detect ATG5 phosphorylation. (H) LN229 cells with or without shPAK1 or WT HA-PAK1 or HA-PAK1K420R transfection were subjected to hypoxia. A specific anti-p-ATG5 (T101) antibody produced by our group was used to detect ATG5 phosphorylation. (I) Before hypoxia treatment, PAK1-depleted LN229 cells with the reintroduction of WT HA-PAK1 or HA-PAK1K229A mutant were subjected to FRAX597 treatment or not for 1 hr. ATG5 (T101) phosphorylation was measured as indicated
