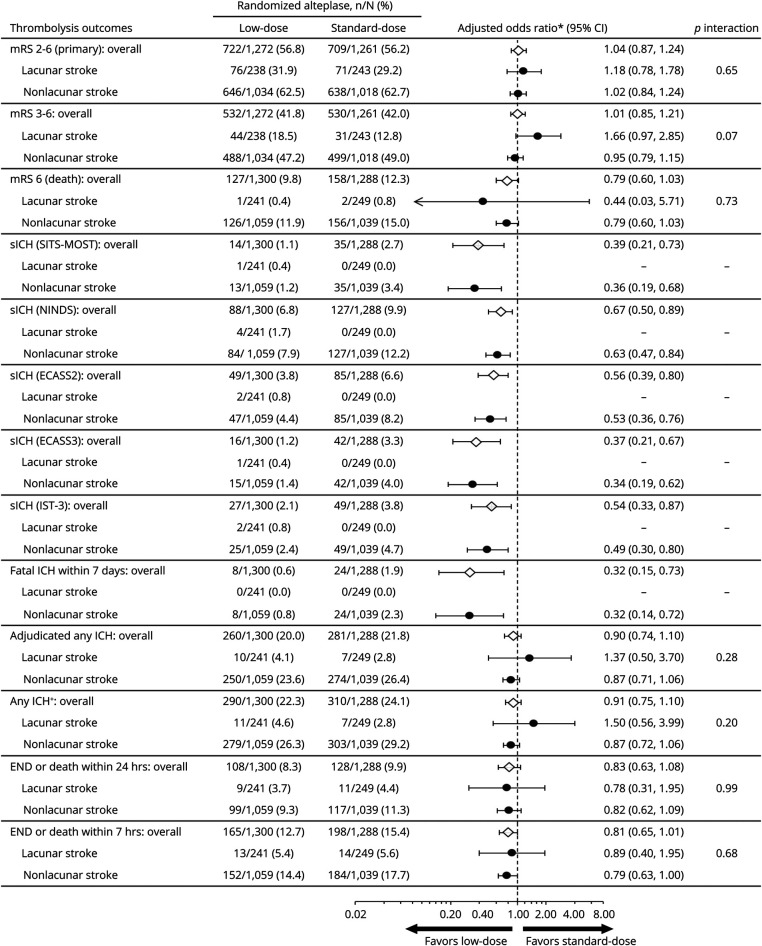
Figure 3. Thrombolysis Outcomes in Participants With Definite/Probable Lacunar and Nonlacunar Stroke by Randomized Treatment.
*Adjusted for key prognostic covariates (age, sex, ethnicity, baseline NIH Stroke Scale [NIHSS] score, time from stroke onset to randomization, premorbid function [modified Rankin Scale (mRS) scores 0 or 1], prior use of antithrombotic agents [aspirin, other antiplatelet agent, or warfarin], history of diabetes or cardiovascular disease [stroke, atrial fibrillation, coronary artery disease, valvular or other heart disease], assigned to intensive blood pressure–lowering group) for functional outcomes. Adjusted for minimization and key prognostic covariates (age, baseline NIHSS score, time from stroke onset to randomization, and assigned to intensive blood pressure–lowering group) for safety outcomes and neurologic deterioration within 24 hours or 7 days.†Site reported or adjudicated centrally. CI = confidence interval; ECASS = European–Australian Cooperative Acute Stroke Study; END = early neurologic deterioration; ICH = intracerebral hemorrhage; IST-3 = third International Stroke Trial; NINDS = National Institutes of Neurologic Diseases and Stroke; sICH = symptomatic intracerebral hemorrhage; SITS-MOST = Safe Implementation of Thrombolysis in Stroke–Monitoring Study.