Figure 6. NOX2-dependent ROS are necessary for load-induced sclerostin degradation and bone formation in vivo.
(A) Dissected ulnae and radii flushed of marrow were treated with hydrogen peroxide (100 μM) as a source of ROS for 5 min before homogenization. Western blots were probed with sclerostin and β-actin (n = 6 mice). (B) Thirteen week old male C57Bl/6 mice treated with vehicle (saline, n = 10 mice) or apocynin (3 mg/kg, n = 8 mice) to inhibit NOX2 were forearm loaded (1800 με, 90 s, 2 Hz) and labeled with calcein and alizarin red at the indicated times for dynamic histomorphometry. Representative periosteal double labeling is shown. (C) Periosteal bone formation rate (Ps.BFR) and (D) periosteal mineral apposition rate (Ps.MAR) were calculated. (E) Fourteen to 17 week old male and female C57Bl/6 mice treated with vehicle (saline + 4% DMSO, i.p., n = 14) or apocynin (3 mg/kg in saline, i.p., n = 12 mice) to inhibit NOX2 were treated 2 hr prior to ulnar loading (2000 με, 90 s, 2 Hz). Non-loaded and loaded limbs were isolated 5 min post-load, and western blots were probed for sclerostin and β-actin. Vehicle data is duplicated in Figure 7D as all animals were run and processed together. Graphs depict mean ± SD. *p<0.05, **p<0.01 by unpaired two-tailed t-test (A), two-way ANOVA with Holm–Sidak post hoc correction (C, D), or Kruskal–Wallis with Dunn’s post hoc correction (E).
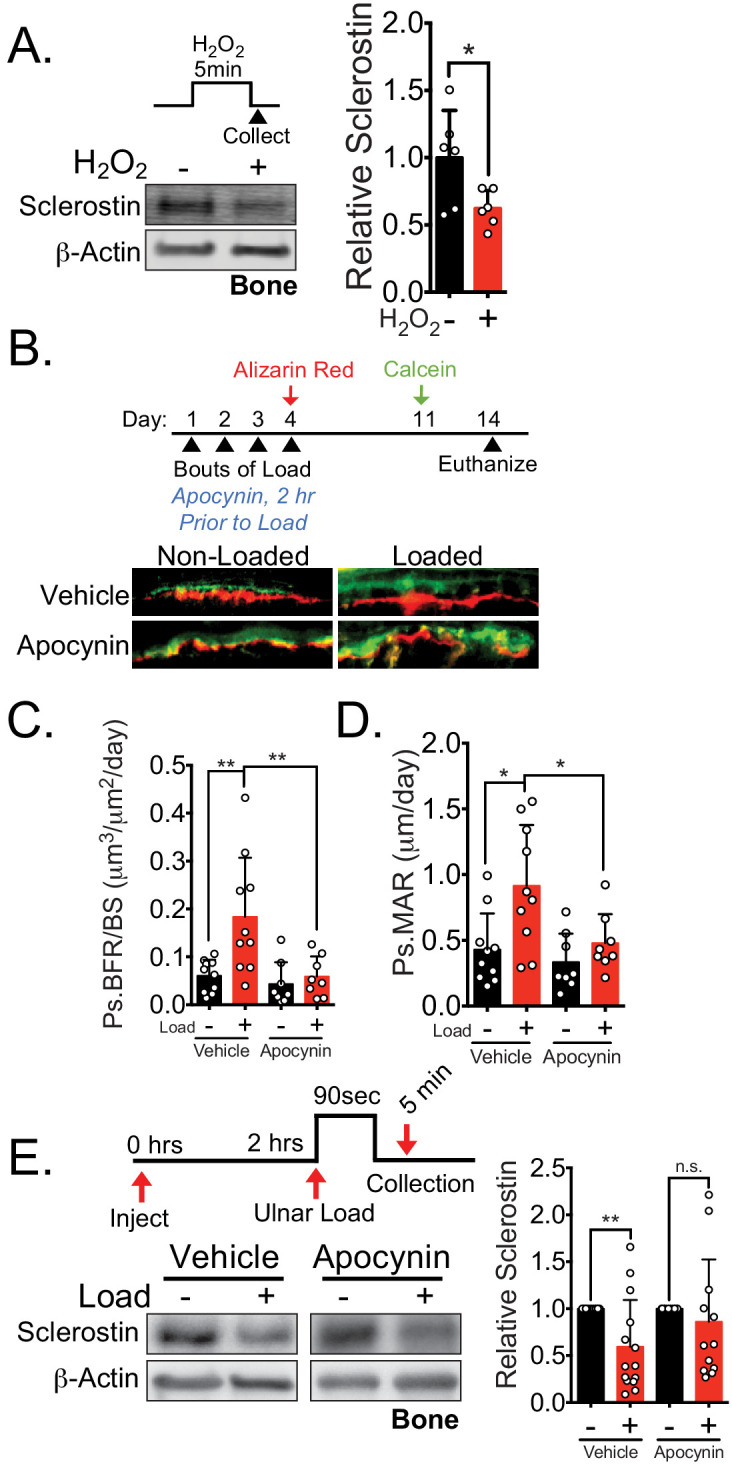
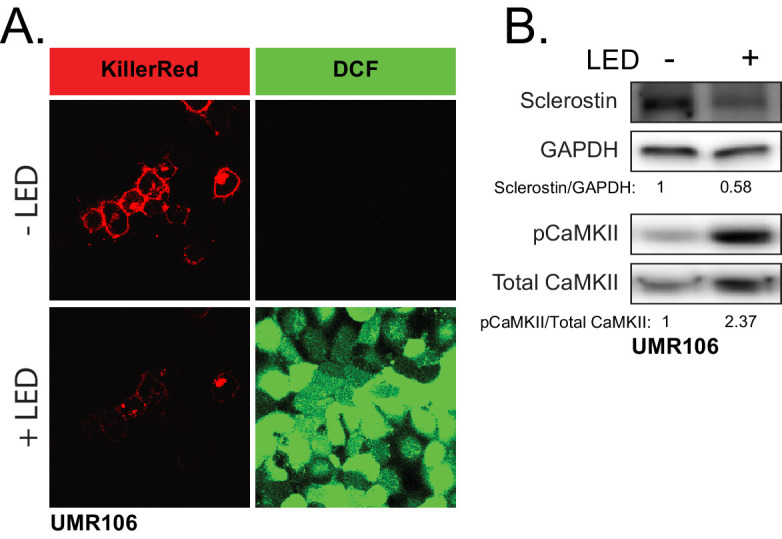
Figure 6—figure supplement 1. ROS is sufficient to drive CaMKII activation and loss of sclerostin protein.