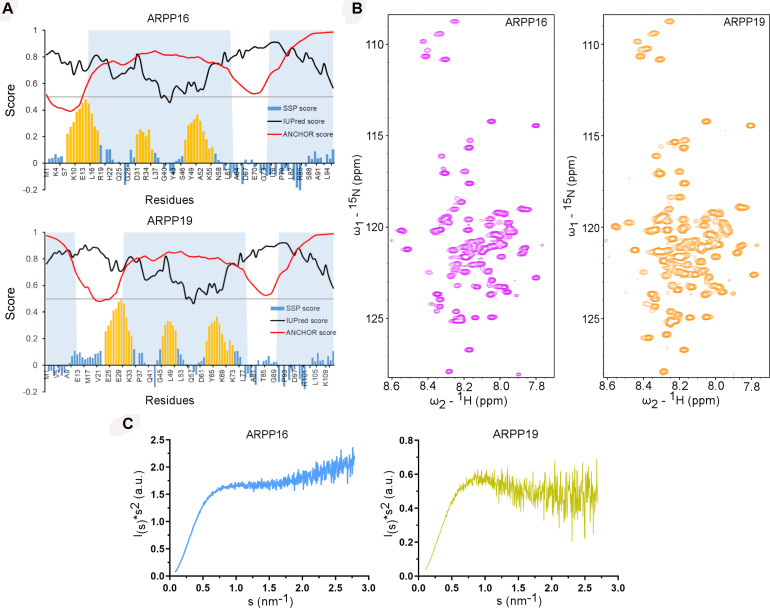
FIGURE 2.
ARPP-19 and ARPP-16 are intrinsically disordered proteins having propensity to form three transient α-helices. (A) IUPRED2A analysis and secondary structure propensity (SSP) calculation of ARPPs. IUPred2 and Anchor2 score are represented with black and red curves, respectively. The disordered prediction score in black highlights disordered nature of ARPPs and disordered binding region prediction score in red suggests presence of multiple disordered binding regions which are shown as shaded region. SSP score for ARPPs calculated using 1Hα, 13Cα, and 13Cβ chemical shift shows that both ARPPs have propensity to form three α-helices. The positions of α-helices are highlighted with orange color. (B) The cross peaks in the 2D 15N-HSQC spectra of uniformly 15N labeled ARPPs are highly overlapped because of the degeneracy of the proton spectra dispersion indicating that the ARPPs lacks well-defined 3D structure. (C) The Kratky plot calculated from SAXS data exhibits rising curve with increasing angle, which corresponds to the scattering pattern of IDPs.