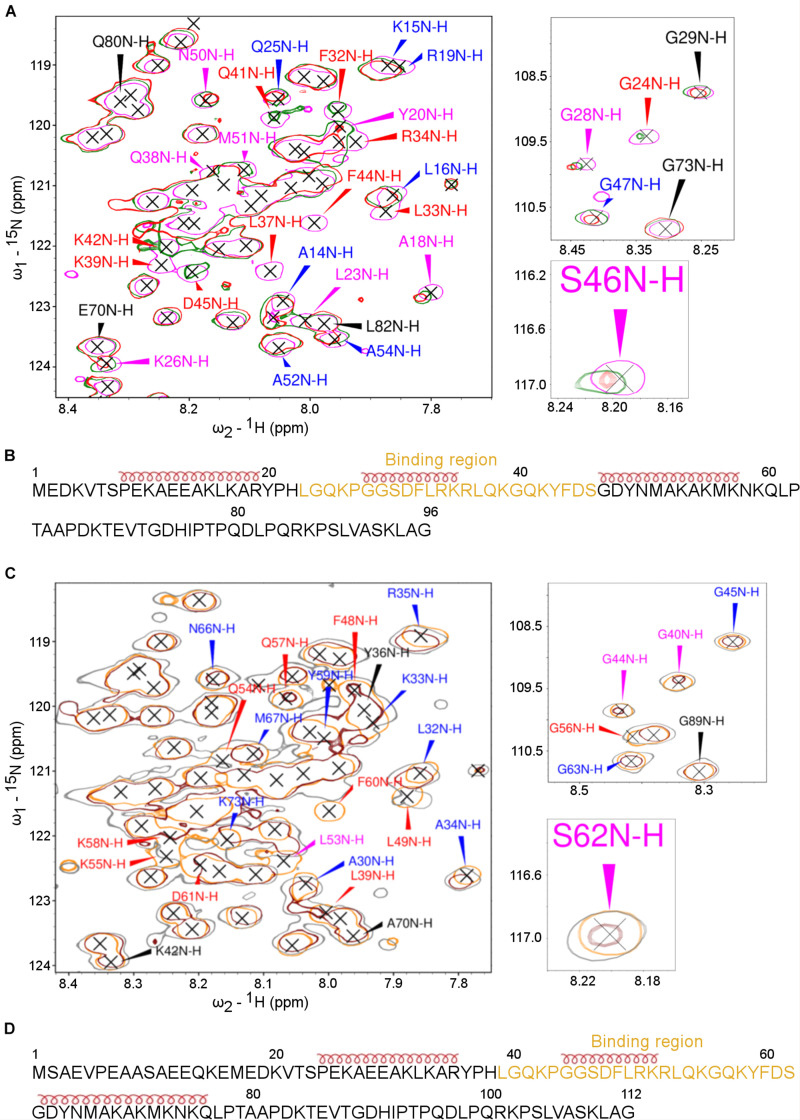
FIGURE 8.
The PP2A A-subunit binds to the 2nd transient α-helices. HSQC spectra of 15N-labeled ARPPs collected before and after the addition of the PP2A A-subunit reveals the A-subunit interacts with the region that based on SSP calculations form a transient α-helices. (A) The overlaid of 15N-HSQC of 15N-labeled free ARPP-16 (magenta), 1:1 (green), 1:2 (red) ARPP-16: PP2A A-subunit. The NH cross peaks that broadened with 1:2 PP2A A-subunit-ARPP-16 are labeled red, whereas the cross peaks that shifted the most and exhibit significant line broadening are labeled with magenta and the NH correlations with small CSPs are labeled blue. Those NH cross peaks whose intensity and position remained intact are labeled with black color. (B) ARPP-16 sequence showing the epitope that interacts with the A-subunit of PP2A as well as the positions of transient α-helices obtained from SSP calculations are shown. (C) The overlaid of 15N-HSQC of 15N-labelled free ARPP-19 (orange), 1:1 (gray) and 1:2 (maroon) ratio with PP2A A-subunit. The effect to the addition of 1:2 ratio of A-subunit into ARPP-19 are labeled similarly as in panel (A). (D) ARPP-16 sequence showing the region that interacts with the A-subunit of PP2A as well as the positions of the transient α-helices obtained from SSP calculations.