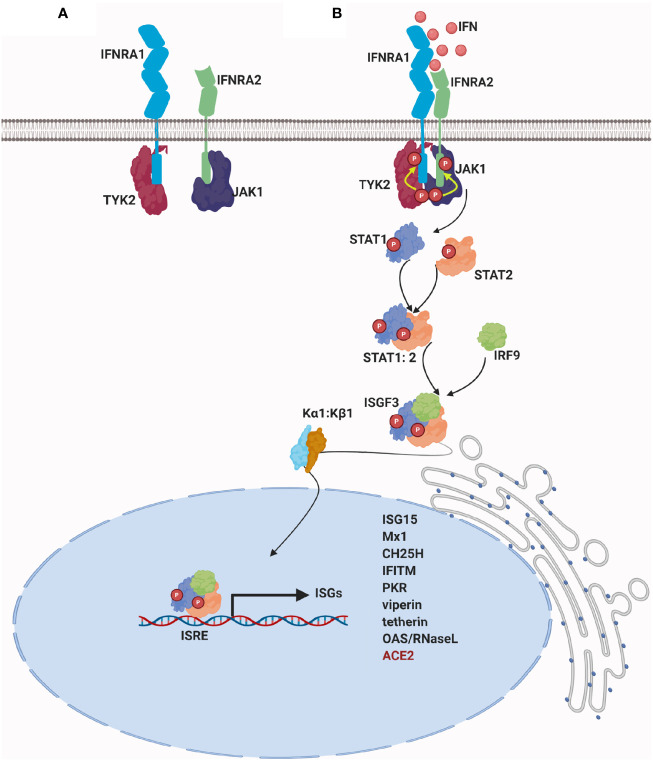
Figure 2.
Signaling of type I IFN: (A) In the absence of a stimulus, a specific JAK protein in an inactive conformation binds constitutively to the cytoplasmic domain of each IFN receptor chain: IFNAR1 associates with tyrosine kinase 2 (Tyk2) and IFNAR2 associates with tyrosine kinase Janus 1(JAK1) (36). (B) Upon IFN binding, receptor chains are brought into close proximity, and the two JAK kinase domains remain juxtaposed and undergo transphosphorylation and sustained activation. Once activated, JAKs phosphorylate IFN receptor´s chains on highly conserved tyrosine residues, which lead to the repositioning or binding of STAT proteins (37). As a result, STATs are phosphorylated on conserved tyrosine residues and they are dissociated from the receptor (21). Structural changes of activated STATs leads to heterodimerization of STAT1 and STAT2 and to its interaction with IFN regulatory factor 9 (IRF9). Binding of STAT1, STAT2, and IRF9 results in the formation of a complex called IFN-stimulated gene factor 3 (ISGF3) (38–40). ISGF3 complex is normally translocated into the nucleus by binding to karyopherin alpha 1 (KPNA1) and recruits karyopherin beta 1(KPNB1) (41). ISGF3 binds IFN-stimulated regulatory elements (ISREs) in the DNA upstream of IFN-stimulated genes (ISGs), resulting in the transcription of hundreds of type I ISGs. For further details visit (10, 11, 19, 21, 42).