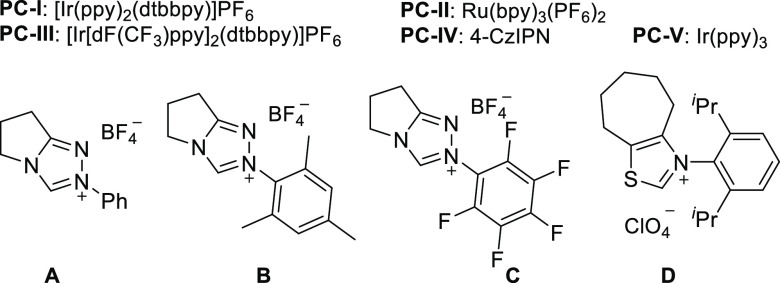
Table 1. Reaction Optimizationa.
| entry | PC | NHCb | sulfinate | base (equiv) | yield of 3a (%)c |
|---|---|---|---|---|---|
| 1 | PC-I | A | PhSO2Na | Cs2CO3 (2) | n.d. |
| 2 | PC-I | A | PhSO2Na | KOtBu (2) | n.d. |
| 3 | PC-I | A | PhSO2Na | DBU (2) | n.d. |
| 4 | PC-I | A | PhSO2Na | Cs2CO3 (1) + KOtBu (1) | trace |
| 5 | PC-I | A | PhSO2Na | Cs2CO3 (1) + DBU (1) | 8 |
| 6 | PC-I | A | PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 26 |
| 7 | PC-I | B | PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 39 |
| 8 | PC-I | C | PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 17 |
| 9 | PC-I | D | PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 22 |
| 10 | PC-II | B | PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 43 |
| 11 | PC-III | B | PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 28 |
| 12 | PC-IV | B | PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 35 |
| 13 | PC-V | B | PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | n.d. |
| 14 | PC-II | B | 4-OMe-PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 10 |
| 15 | PC-II | B | 4-Cl-PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 56 |
| 16 | PC-II | B | 4-CN-PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 48 |
| 17 | PC-II | B | MeSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 4 |
| 18 | PC-II | B | 4-Cl-PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | 81 (78)d |
| 19 | PC-II | B | 4-Cl-PhSO2Na | Cs2CO3 (0.5) + MTBD (1.3) | n.d.e |
Reaction conditions: 1a (0.15 mmol), 2a (0.3 mmol), NHC (15 mol %), photoredox catalyst (1.5 mol %), PhSO2Na (25 mol %), base (2.0 equiv), and MeCN (1.5 mL) under irradiation with a 23 W CFL for 24 h.
GC yields using biphenyl as an internal standard. The yield of the isolated product is given in parentheses.
20 mol % NHC, 30 mol % 4-Cl-PhSO2Na, and 2.5 equiv of 2a were applied.
Without photoredox catalyst, NHC, sulfinate, or irradiation.