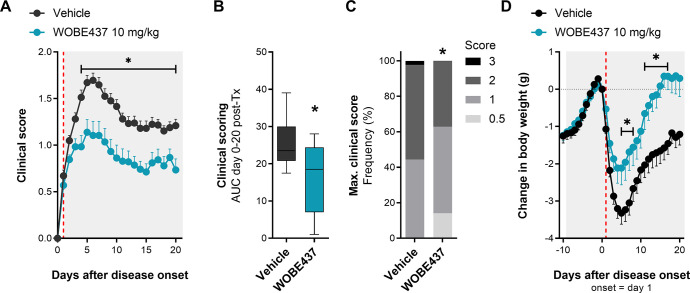
Figure 1.
Chronic treatment with WOBE437 reduced disease severity in EAE female C57BL/6 mice. (A) Time course of clinical score from day 0 to day 20; onset is represented as day 1 (red line). (B) AUC and (C) maximal clinical score observed from the time course of clinical score, from day 0 to day 20. (D) Time course of body weight changes from day −10 to day 20; onset is represented at day 1 (red line). Administration of vehicle (DMSO, n = 37) or WOBE437 (10 mg/kg, n = 29) started at the individual day of onset of each mouse; injections (20 μL) were done intraperitoneally once per day during 20 days. Data show (A, D) mean ± SEM; (B) median, percentile 25, percentile 75, minimum and maximum; or (C) cumulative frequency. (A–D) Data show the summary of four different cohorts, only mice showing symptoms were included in the study. Statistical differences were determined using (A, D) multiple t-tests corrected for multiple comparison with the Holm–Sidak method, (B) Mann–Whitney test, or (C) Chi-square test and Fisher’s exact test. *, p < 0.05 compared to vehicle group.