Abstract
In this secondary analysis of a dose‐titration study of patients with hypertension uncontrolled on prior monotherapy, blacks (n=234) and non‐blacks (n=765) were switched to amlodipine (AML)/olmesartan medoxomil (OM) 5/20 mg, with uptitration every 4 weeks to AML/OM 5/40 mg and then AML/OM 10/40 mg to achieve a seated cuff blood pressure (SeBP) of <120/70 mm Hg. Hydrochlorothiazide 12.5 and 25 mg could be added if SeBP was ≥125/75 mm Hg. The cumulative proportions of patients achieving systolic SeBP <140 mm Hg (<130 mm Hg if diabetic) at 12 weeks were 71.6% for blacks and 77.2% for non‐blacks. Mean SeBP change from baseline in blacks (mean baseline BP: 153.0/93.7 mm Hg) ranged from −11.7/−6.1 mm Hg for AML/OM 5/20 mg to −23.6/−12.9 mm Hg for AML/OM 10/40 mg +hydrochlorothiazide 25 mg (all P<.0001). Antihypertensive efficacy was maintained throughout the 24‐hour dosing interval. An AML/OM‐based regimen was effective in blacks with hypertension uncontrolled on prior monotherapy.
Hypertension is more common among black individuals in the United States than in other ethnic groups, with an age‐adjusted prevalence rate of 40% compared with 27% for whites.1 In addition, hypertension among black Americans is of greater severity and has an earlier onset, resulting in higher rates of cardiovascular disease (CVD) compared with non‐Hispanic whites.1, 2, 3 For example, rates for nonfatal stroke (1.3‐fold), fatal stroke (1.8‐fold), heart disease death (1.5‐fold), and end‐stage kidney disease (4.2‐fold) are higher among blacks compared with whites.3
The pattern of hypertension among black individuals in Africa is somewhat different. A study analyzing data from 17 population studies in sub‐Saharan African countries indicated an overall prevalence rate of 16.2% for 2008, with higher rates among urban than rural populations.4 The rural/urban difference was highlighted by data that demonstrate that migration from rural to urban areas and from African countries to the United States is associated with an increase in blood pressure (BP) among black individuals.5
The increased prevalence of hypertension and adverse clinical outcomes among blacks is likely related to a combination of genetic and environmental factors.6 For example, black patients, particularly in the southeastern United States, have a high prevalence of patient‐related risk factors for CVD such as obesity, diabetes mellitus, and hypercholesterolemia, and these risk factors are frequently clustered.7 Blacks also appear to have greater vascular resistance and/or disturbances in both endothelial‐dependent and ‐independent vasodilation compared with other populations.6
The Blood Pressure Control in All Subgroups With Hypertension (BP‐CRUSH) study was designed to evaluate improvements in BP control among patients previously uncontrolled on antihypertensive monotherapy who were switched to fixed‐dose amlodipine/olmesartan medoxomil (AML/OM) ±hydrochlorothiazide (HCTZ) combination therapy.8 This article reports the effectiveness of the AML/OM‐based treatment regimen in reducing BP and achieving BP goals in prespecified subgroups of black and non‐black patients in the BP‐CRUSH study.
Methods
Study Design
The BP‐CRUSH study (ClinicalTrials.gov identifier: NCT00791258) was a phase IV (IIIb in South Africa), prospective, open‐label, multicenter, single‐arm, dose‐titration study. The study included a 20‐week active treatment period in patients with hypertension who had uncontrolled BP on prior monotherapy after 1 month. A complete description of the study design, methods, and main results has been published previously.8
Men and women aged 18 to 80 years were eligible if they had uncontrolled BP (mean systolic BP [SBP] ≥140 mm Hg [or ≥130 mm Hg for patients with diabetes] and ≤180 mm Hg and mean diastolic BP [DBP] ≤110 mm Hg on two consecutive visits during screening) after ≥1 month of antihypertensive monotherapy with an angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker, β‐blocker, calcium channel blocker (CCB), or diuretic.
On study entry, patients who met the inclusion criteria were switched on day 1 from their previous antihypertensive monotherapy (without a washout period) to a fixed‐dose combination of AML/OM 5/20 mg, administered once daily in the morning. Patients were eligible to uptitrate to any AML/OM dose combination if mean SBP was ≥120 mm Hg and <200 mm Hg or if mean DBP was ≥70 mm Hg and <115 mm Hg. Patients were further eligible to uptitrate to an HCTZ‐containing regimen if mean SBP was ≥125 mm Hg and <200 mm Hg or if mean DBP was ≥75 mm Hg and <115 mm Hg. Uptitration was permitted every 4 weeks to AML/OM 5/40 mg, AML/OM 10/40 mg, AML/OM 10/40 mg +HCTZ 12.5 mg, and AML/OM 10/40 mg +HCTZ 25 mg according to the schedule in Figure 1. If patients achieved adequate BP control, they were maintained at their current drug dose. However, if their BP became uncontrolled during the maintenance phase (SBP ≥130 mm Hg or DBP ≥80 mm Hg), patients were uptitrated to the next dosage level and re‐entered into the titration phase of the study. Patients receiving AML/OM 10/40 mg (±HCTZ) who achieved a mean SBP of <120 mm Hg and mean DBP of <70 mm Hg, and who were asymptomatic for hypotension, entered maintenance treatment and continued in the study at their current dose. Patients with a mean SBP of ≥200 mm Hg or DBP of ≥115 mm Hg at any visit exited the study, as did patients with either an SBP <120 mm Hg or DBP <70 mm Hg with symptomatic hypotension.
Figure 1.
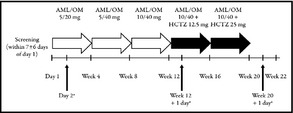
Study design. AML indicates, amlodipine; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil. aScheduled ambulatory blood pressure monitoring (ABPM) measurement for ABPM cohort. Reprinted from Neutel J, Shojaee A, Maa J‐F. Efficacy of amlodipine/olmesartan ± hydrochlorothiazide in patients uncontrolled on prior calcium channel blocker or angiotensin II receptor blocker monotherapy. Adv Ther. 2012;29(6):508–523, with kind permission from Springer Science+Business Media B.V.
Study Assessments
At each visit during active treatment, the average of 3 seated cuff BP (SeBP) values was measured using an automated BP device (Omron HEM‐705CP; Omron Corporation, Tokyo, Japan). At baseline and weeks 12 and 20, 24‐hour ambulatory BP monitoring (ABPM) was performed in 243 patients at 39 selected sites in the United States.
The primary efficacy endpoint was the cumulative percentage of patients achieving the seated cuff SBP (SeSBP) goal of <140 mm Hg (or <130 mm Hg in patients with diabetes) during the first 12 weeks of active treatment. Secondary endpoints included the cumulative percentage of patients achieving the SeBP goal of <140/90 mm Hg (or <130/80 mm Hg for patients with diabetes) during weeks 12 and 20 of treatment; mean change from baseline in SeSBP and seated cuff DBP (SeDBP) during each dose‐titration period; the percentage of patients achieving the SeBP threshold of <140/90 mm Hg during each dose‐titration period; changes from baseline in mean ambulatory BP over 24 hours and during the daytime (8 am–4 pm), nighttime (10 pm–6 am), and final 2, 4, and 6 hours of the dosing interval after 12 and 20 weeks of treatment; and achievement of American Heart Association–recommended ABPM targets of <130/80 mm Hg for mean 24‐hour ABPM, <135/85 mm Hg for mean daytime ABPM, and <120/70 mm Hg for mean nighttime ABPM.9
Safety assessments included the evaluation of adverse events (AEs), laboratory parameters, and physical examination. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA, Chantilly, VA) version 12.0.
Statistical Analysis
The treated study population included all patients who received ≥1 dose of study medication, while the ABPM subgroup included all treated patients with valid baseline and week 12 or 20 ABPM data. Summary statistics were calculated for all continuous and categorical variables. The cumulative proportion of patients achieving the SeBP goal by titration dose was calculated as the ratio of the number of patients who achieved the goal at any time from the first dose date to the end of the titration dose period date over the number of patients who had any postbaseline BP data by the end of the titration dose period. Changes in SeBP and ambulatory BP from baseline were summarized by titration dose (last observation carried forward [LOCF] method) and by visit without the LOCF method, and analyzed by the one‐sample paired t test performed at a 2‐sided significance level of 5%.
Results
Demographics and Baseline Characteristics
In the overall study, a total of 999 patients entered active treatment, including 234 blacks and 765 non‐blacks. In the ABPM subgroup, there were 60 and 229 blacks and non‐blacks, respectively. Patient demographics are summarized in Table 1. Overall, the proportions of blacks and non‐blacks with type 2 diabetes mellitus were similar; however, a greater proportion of non‐blacks had metabolic syndrome vs blacks (48.5% vs 38.9%, respectively). Both low‐density lipoprotein and high‐density lipoprotein cholesterol levels were similar between the cohorts, whereas a greater proportion of non‐blacks had higher triglyceride levels. Creatinine levels were also similar between both cohorts; however, the mean glomerular filtration rate (GFR) was lower in non‐blacks compared with blacks (83.3 vs 97.0 mL/min/1.73 m2, respectively).
Table 1.
Demographics and Baseline Characteristics
| Characteristics | Blacks (n=234) | Non‐Blacks (n=765) |
|---|---|---|
| Age, y, mean (±SD) | 52.6 (10.7) | 56.6 (11.5) |
| ≥65 y, No. (%) | 33 (14.1) | 195 (25.5) |
| Female, No. (%) | 137 (58.5) | 354 (46.3) |
| Weight, kg, mean (±SD) | 90.1 (20.9) | 87.6 (21.6) |
| Body mass index, kg/m2,mean (±SD) | 32.2 (6.7) | 30.7 (6.2) |
| Race, No. (%) | ||
| Caucasian | 0 (0.0) | 630 (82.4) |
| Black | 234 (100.0) | 0 (0.0) |
| Asian | 0 (0.0) | 129 (16.9) |
| American Indian/Alaskan native | 0 (0.0) | 6 (0.8) |
| Ethnicity, No. (%) | ||
| Hispanic or Latino | 4 (1.7) | 101 (13.2) |
| Type 2 diabetes mellitus, No. (%) | 44 (18.8) | 148 (19.3) |
| Metabolic syndrome , a No. (%) | 91 (38.9) | 371 (48.5) |
| Glucose, mg/dL, mean (±SD) | 99.1 (18.3) | 106.1 (22.4) |
| LDL, mg/dL, mean (±SD) | 120.0 (35.0) | 123.0 (34.2) |
| HDL, mg/dL, mean (±SD) | 54.9 (16.7) | 52.8 (16.6) |
| Triglycerides, mg/dL, mean (±SD) | 122.0 (63.2) | 167.2 (100.7) |
| Calculated GFR, mL/min, mean (±SD) | 97.0 (23.4) | 83.3 (19.7) |
| Creatinine, mg/dL, mean (±SD) | 1.02 (0.23) | 1.02 (0.22) |
| SeSBP, mm Hg, mean (±SD) | 153.0 (9.3) | 153.9 (9.1) |
| SeDBP, mm Hg, mean (±SD) | 93.7 (8.5) | 91.4 (8.6) |
| ABPM subgroup, No. | 60 | 229 |
| 24‐H ambulatory BP, mm Hg, mean (±SD) | 137.3 (12.7)/84.0 (10.6) | 135.3 (11.4)/80.3 (8.8) |
Abbreviations: ABPM, ambulatory BP monitoring; GFR, glomerular filtration rate; LDL, low‐density lipoprotein cholesterol; SD, standard deviation; SeDBP, seated cuff diastolic BP; SeSBP, seated cuff systolic BP. aMetabolic syndrome is defined as the presence of ≥3 of the following: high‐density lipoprotein (HDL) cholesterol <50 mg/dL in women and <40 mg⁄dL in men, triglycerides ≥150 mg⁄dL, blood pressure (BP) ≥130/85 mm Hg, or fasting glucose ≥100 mg⁄dL.
Efficacy
Seated Cuff BP
The cumulative proportions of blacks and non‐blacks who achieved the primary endpoint of an SeSBP <140 mm Hg (or <130 mm Hg for patients with diabetes) at 12 weeks was 71.6% (166/232; mean baseline SeSBP, 153.0 mm Hg) and 77.2% (581/753; mean baseline SeSBP, 153.9 mm Hg), respectively. Achievement of this goal increased over the course of the study, with 46.5% and 56.7% of blacks at goal by 4 and 8 weeks, respectively. By comparison, 49.4% and 64.9% of non‐blacks reached this goal by 4 and 8 weeks, respectively.
Mean SeSBP was significantly reduced from baseline at the week 12 and 20 visits in the black (18.4 and 25.5 mm Hg, respectively; both P<.0001) and non‐black (22.8 and 27.2 mm Hg; both P<.0001) subgroups. Mean changes from baseline in SeSBP and SeDBP at the end of each titration dose period in blacks and non‐blacks are summarized in Figure 2. Mean (±standard error) changes from baseline in SeSBP/SeDBP during the titration periods ranged from −11.7 (±0.8)/−6.1 (±0.5) mm Hg with AML/OM 5/20 mg to −23.6 (±1.4)/−12.9 (±0.9) mm Hg with AML/OM 10/40 mg +HCTZ 25 mg for blacks and from −15.0 (±0.5)/−8.2 (±0.3) mm Hg to −25.7 (±0.7)/−14.1 (±0.5) mm Hg, respectively, for non‐blacks. The reductions from baseline in SeBP at the end of each titration dose period were statistically significant (P<.0001) at all dose levels for both blacks and non‐blacks.
Figure 2.
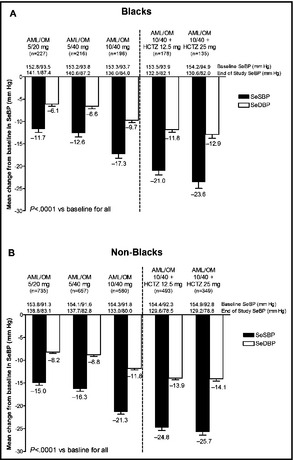
Mean±standard error change from baseline in seated cuff systolic blood pressure (SeSBP) and seated cuff diastolic blood pressure (SeDBP) at the end of each titration dose period in (A) blacks and (B) non‐blacks. AML indicates amlodipine; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SeBP, seated cuff blood pressure.
The proportion of patients achieving the SeBP threshold of <140/90 mm Hg at the highest dual combination (AML/OM 10/40 mg) and triple combination (AML/OM 10/40 +HCTZ 25 mg) therapy doses in the black subgroup was 68.7% and 87.2%, respectively, and was 79.7% and 91.2% in the non‐black subgroup. Achievement of SeBP thresholds increased with dose titration. Cumulatively, SeBP threshold (<140/90 mm Hg) achievement ranged from 45.4% to 87.2% for blacks across the AML/OM‐based dosing regimen (Figure 3). For non‐blacks, the cumulative proportions of patients achieving the SeBP threshold (<140/90 mm Hg) ranged from 50.8% to 91.2% across the AML/OM‐based dosing regimen (Figure 3).
Figure 3.
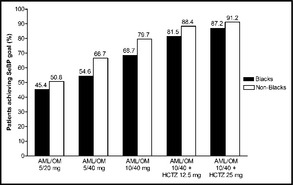
Cumulative proportions of blacks and non‐blacks achieving the seated cuff blood pressure (SeBP) threshold of <140/90 mm Hg by titration dose. AML indicates amlodipine; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil.
24‐Hour ABPM
Among the subgroup of blacks who underwent ABPM, mean 24‐hour, mean daytime, and mean nighttime ambulatory BP values were all significantly decreased from baseline at weeks 12 and 20 (P<.0001 for all comparisons; Figure 4a and 4b). At week 20, the mean (±standard error) 24‐hour change in ambulatory BP was −23.1 (±2.01)/−15.0 (±1.33) mm Hg among blacks and −20.5 (±0.93)/−12.9 (±0.61) mm Hg among non‐blacks. Mean hourly ambulatory SBP levels at baseline, week 12, and week 20 are summarized in Figure 5a and 5b for blacks and non‐blacks, respectively.
Figure 4.
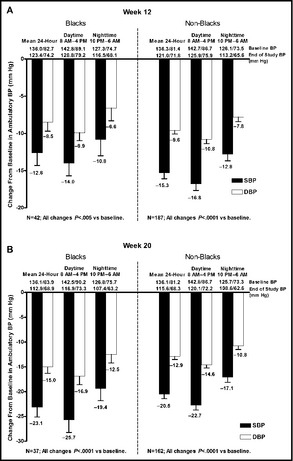
Mean change from baseline in mean 24‐hour ambulatory blood pressure (BP) at (a) week 12 and (b) week 20 in blacks and non‐blacks. DBP indicates diastolic BP; SBP, systolic BP.
Figure 5.
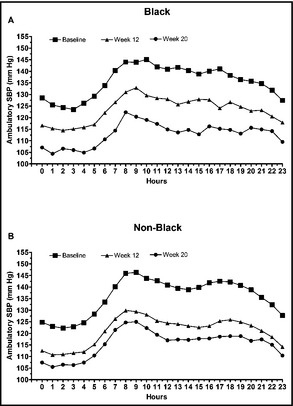
Mean hourly ambulatory systolic blood pressure (SBP) at baseline, week 12, and week 20 in (a) blacks and (b) non‐blacks.
Ambulatory SBP was controlled throughout the 24‐hour dosing period in both black and non‐black cohorts at week 20. BP control was maintained throughout the dosing period, with sustained decreases in BP in the final 2, 4, and 6 hours of the dosing interval. At week 20, mean changes from baseline in ambulatory BP during the last 6 hours to the last 2 hours ranged from −20.9/−13.8 mm Hg to −24.0/−15.3 mm Hg for blacks, respectively, and from −17.2/−10.8 mm Hg to −18.6/−11.6 mm Hg among non‐blacks. Decreases from baseline in ambulatory BP at week 20 were statistically significant (P<.0001) at all measured intervals (ie, 24 hours, daytime, nighttime, and last 2, 4, or 6 hours of dosing). It should be noted that patients in the black cohort had higher mean ambulatory BP values at baseline compared with non‐black patients for the final 6, 4, and 2 hours of the dosing interval (131.6/79.5 vs 129.1/76.5 mm Hg, 134.3/81.9 vs 132.2/78.8 mm Hg, and 139.2/86.4 vs 138.1/83.6 mm Hg, respectively), as well as higher median ambulatory BP values at baseline (135.1/80.4 vs 127.7/76.9 mm Hg, 136.6/84.7 vs 131.6/78.8 mm Hg, and 138.4/86.5 mm Hg vs 136.7/84.0 mm Hg).
Achievement of ambulatory BP targets at weeks 12 and 20 tended to be lower in blacks compared with non‐blacks. Among blacks, 61.9% achieved the mean 24‐hour ambulatory BP target (<130/80 mm Hg) at week 12, while 91.9% achieved the target at week 20. For non‐blacks, 75.9% and 90.1% achieved this target at weeks 12 and 20, respectively. The daytime target (<135/85 mm Hg) was achieved in 59.5% of blacks at week 12 and in 86.5% at week 20. For non‐blacks, the daytime target was achieved in 75.9% of patients at week 12 and in 88.9% of patients at week 20. The nighttime target (<120/70 mm Hg) was achieved in 50.0% of blacks at week 12 and in 75.7% at week 20. The corresponding nighttime values for non‐blacks were 64.7% and 79.6%.
Safety and Tolerability
Overall, treatment was generally well tolerated in black and non‐black patient cohorts. The proportion of blacks who developed any treatment‐emergent AEs (TEAEs) ranged from 14.0% to 27.9% across doses (Table 2). By comparison, TEAEs were observed in 17.5% to 25.0% across doses for non‐blacks. There was no apparent effect of an AML/OM dose or the addition of HCTZ on the occurrence of AEs. The most frequently reported AEs (dizziness and peripheral edema) were similar in blacks and non‐blacks. All drug‐related AEs were mild to moderate in severity. There was one serious TEAE in the black subgroup and 11 in the non‐black subgroup; however, none of these events were considered to be drug‐related.
Table 2.
Summary of AEs
| AE, No. (%) | AML/OM 5/20 mg | AML/OM 5/40 mg | AML/OM 10/40 mg | AML/OM 10/40 mg +HCTZ 12.5 mg | AML/OM 10/40 mg +HCTZ 25 mg |
|---|---|---|---|---|---|
| Blacks, No. | 234 | 219 | 199 | 183 | 136 |
| Patients with any TEAE a | 45 (19.2) | 41 (18.7) | 47 (23.6) | 51 (27.9) | 19 (14.0) |
| Patients with any drug‐related TEAE | 14 (6.0) | 8 (3.7) | 13 (6.5) | 16 (8.7) | 7 (5.1) |
| Patients with any serious TEAE | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Individual drug‐related TEAEs in >1% of patients | |||||
| Dizziness | 3 (1.3) | 1 (0.5) | 0 (0.0) | 6 (3.3) | 1 (0.7) |
| Peripheral edema | 1 (0.4) | 2 (0.9) | 5 (2.5) | 1 (0.5) | 0 (0.0) |
| Blood creatinine increased | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 2 (1.5) |
| Headache | 3 (1.3) | 2 (0.9) | 2 (1.0) | 2 (1.1) | 0 (0.0) |
| Edema | 0 (0.0) | 0 (0.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) |
| Non‐blacks, No. | 765 | 673 | 596 | 516 | 360 |
| Patients with any TEAE a | 169 (22.1) | 117 (17.4) | 141 (23.7) | 129 (25.0) | 80 (22.2) |
| Patients with any drug‐related TEAE | 61 (8.0) | 31 (4.6) | 57 (9.6) | 58 (11.2) | 40 (11.1) |
| Patients with any serious TEAE | 2 (0.3) | 2 (0.3) | 3 (0.5) | 3 (0.6) | 1 (0.3) |
| Individual drug‐related TEAEs in >1% of patients | |||||
| Peripheral edema | 12 (1.6) | 6 (0.9) | 30 (5.0) | 6 (1.2) | 4 (1.1) |
| Dizziness | 17 (2.2) | 9 (1.3) | 11 (1.8) | 20 (3.9) | 12 (3.3) |
| Blood uric acid increased | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 6 (1.7) |
| Fatigue | 9 (1.2) | 3 (0.4) | 2 (0.3) | 4 (0.8) | 2 (0.6) |
| Hypotension | 3 (0.4) | 5 (0.7) | 4 (0.7) | 4 (0.8) | 4 (1.1) |
Abbreviations: AE, adverse event; AML, amlodipine; HCTZ, hydrochlorothiazide; OM, olmesartan medoxomil; SAE, serious AE. aTreatment‐emergent AE (TEAE) is any AE that either first occurred on/after the first active dose date, or occurred before the first active dose date, then occurred again during active treatment with worsened severity, and occurred no later than 14 days after the last active dose.
Discussion
Black individuals have a higher mean BP and a higher prevalence of uncontrolled hypertension compared with whites.10, 11 Paradoxically, although African Americans are more likely to be aware of their hypertension and more likely to receive antihypertensive therapy, they are less likely to achieve BP targets compared with whites.1, 3 A number of factors likely influence decreased BP control among blacks.1, 12 These factors include a poorer quality of diet intake, higher rates of overweight/obesity, lower physical inactivity, higher psychosocial stress, poorer access to healthcare, and an increased prevalence of some gene polymorphisms.13, 14 Among the physiologic factors that contribute to the disparity in hypertension in blacks is the increased ratio of sodium to potassium intake, due to excess sodium intake and/or an inadequate potassium intake, as well as a modest increase in sodium sensitivity. Nonetheless, there are many physiologic factors such as increased α‐receptor sensitivity, decreased β‐receptor sensitivity, increased endothelin, and a relative reduction in nitric oxide/reactive oxide species ratio.1, 15 In addition, clinical inertia is a common problem among all races, including blacks, which results in undertreatment due to the failure of healthcare workers to increase dosages or add a new medication.12, 16
Despite some underlying differences in the etiology, most clinical trials have demonstrated that antihypertensive therapy is similarly effective for preventing cardiovascular outcomes in blacks compared with non‐blacks. However, drug responses may differ by ethnicity, and there are data to suggest that black patients respond with less of a BP reduction compared with white patients, particularly with renin‐angiotensin system (RAS) blocker treatment. Recent trials show that more than two thirds of patients require ≥1 antihypertensive medication to achieve BP goals.2 Thus, the use of ≥2 antihypertensive agents is recommended, particularly for patients at higher risk,2, 17 including blacks.18, 19
The consensus statement from the International Society on Hypertension in Blacks (ISHIB) states that all classes of antihypertensive agents are effective for lowering BP in blacks; however, data exist that show certain classes of agents (ie, diuretics and CCBs) are more effective than others (ie, RAS inhibitors and β‐blockers) when used as monotherapy.14, 18 The ISHIB consensus statement recommends that combination therapy with ≥2 antihypertensive agents is a rational and preferred therapeutic approach (particularly when SBP is >15 mm Hg and/or DBP is >10 mm Hg above goal), because combining two agents at low doses is more effective than using maximal doses of a single agent and is likely to be associated with fewer AEs.14, 18 For patients who do not achieve target BP with two agents, the dose of one agent can be increased or a third agent can be added.14, 18 Appropriate first‐line combinations include a CCB plus a RAS inhibitor or a thiazide diuretic–based regimen unless there is a compelling indication for other agents.14
Results of the current subanalysis demonstrate that an AML/OM‐based titration regimen is effective for achieving target BP goals in black patients. More than 70% of blacks achieved the primary endpoint of an SeSBP of <140 mm Hg (or <130 mm Hg for patients with diabetes) at week 12. In addition, more than 80% of blacks achieved the SeBP goal of <140/90 mm Hg at week 20. The reduction in BP and achievement of BP targets was clearly greater with higher doses of AML/OM and the addition of HCTZ. Demographics were comparable between the two cohorts, with the exception that non‐blacks had a higher incidence of metabolic syndrome, higher mean triglyceride levels, and a mild decrease in GFR compared with blacks, although mean creatinine levels were similar. There is a persistent difference in the BP control rates comparatively between non‐blacks and blacks. This may be due to the difference in the underlying pathophysiology of the RAS and autonomic nervous system between non‐blacks and blacks.13 However, the control rates achieved in this study exceed those reported for black patients in the National Health and Nutrition Examination Survey (ie, 42.7% of treated blacks).20 The strong relationship between BP control and a reduction in CVD in all populations suggests the potential for improved CVD outcomes in blacks.2
In this study, 24‐hour ABPM demonstrated that the treatment regimen was also associated with significant reductions throughout the daytime and nighttime, with the majority of patients achieving American Heart Association‐recommended ABPM targets. Importantly, ABPM demonstrated that BP reductions were maintained during the last 2 to 6 hours of the dosing interval. Maintenance of antihypertensive efficacy throughout the dosing interval is important, especially during the final hours wherein a morning BP surge is associated with an increased risk of cardiovascular events.21 Although the current treatment regimen resulted in greater ambulatory BP reductions in blacks compared with non‐blacks during the last 2 to 6 hours of the dosing interval, it should be noted that both the mean and median BP baseline values were numerically higher during these time points in the black cohort vs the non‐black cohort, which may have contributed to the greater BP reductions observed in the black cohort. Overall, treatment was well tolerated with no apparent differences between blacks and non‐blacks in the frequency, type, and severity of AEs.
A possible explanation for some of the improvement in BP goal achievement in the study is that physicians were given the freedom to titrate medications at a threshold of <120/70 mm Hg to reach a BP goal of <140/90 mm Hg (<130/80 mm Hg in patients with diabetes). In clinical practice, clinicians are free to titrate medications at any level to reach a recommended goal BP. Similarly, the recent ISHIB consensus statement suggests that decreasing the goal of treatment in patients with target organ disease to 135/85 mm Hg is guided by the goal of shifting the mean BP of the entire population of hypertensive patients to a lower point in blacks.
Conclusions
This subanalysis of the BP‐CRUSH study demonstrates that an AML/OM‐based titration regimen, with or without HCTZ, was effective and well tolerated in blacks and represents a rational treatment option for this patient subgroup.
Acknowledgments and disclosures
This study was supported by Daiichi Sankyo, Inc. Medical writing and editorial services were provided by Bret Fulton and Alan J. Klopp, PhD, of inScience Communications, Springer Healthcare. Shawna Nesbitt, MD, received a grant from Pfizer, served as a consultant for Novartis, Boehringer Ingelheim, and Daiichi Sankyo, Inc, received support for travel, accommodations, and/or meeting expenses from Novartis and Daiichi Sankyo, Inc, and received payments for lectures including service on speaker boards from Novartis, Boehringer Ingelheim, and Daiichi Sankyo, Inc. Ali Shojaee, PharmD, and Jen‐Fue Maa, PhD, are both employees of Daiichi Sankyo, Inc.
J Clin Hypertens (Greenwich). 2013;00:00–00. © 2013 Wiley Periodicals, Inc.
References
- 1. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037–2114. [DOI] [PubMed] [Google Scholar]
- 2. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. [DOI] [PubMed] [Google Scholar]
- 4. Twagirumukiza M, De Bacquer D, Kips JG, et al. Current and projected prevalence of arterial hypertension in sub‐Saharan Africa by sex, age and habitat: an estimate from population studies. J Hypertens. 2011;29:1243–1252. [DOI] [PubMed] [Google Scholar]
- 5. Cooper RS, Amoah AG, Mensah GA. High blood pressure: the foundation for epidemic cardiovascular disease in African populations. Ethn Dis. 2003;13(2 Suppl 2):S48–S52. [PubMed] [Google Scholar]
- 6. Ferdinand KC, Armani AM. The management of hypertension in African Americans. Crit Pathw Cardiol. 2007;6:67–71. [DOI] [PubMed] [Google Scholar]
- 7. Baruth M, Wilcox S, Egan BM, et al. Cardiovascular disease risk factor clustering among African American adults. Ethn Dis. 2011;21:129–134. [PMC free article] [PubMed] [Google Scholar]
- 8. Weir MR, Hsueh WA, Nesbitt SD, et al. A titrate‐to‐goal study of switching patients uncontrolled on antihypertensive monotherapy to fixed‐dose combinations of amlodipine and olmesartan medoxomil ± hydrochlorothiazide. J Clin Hypertens (Greenwich). 2011;13:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 10. Liu X, Liu M, Tsilimingras D, Schiffrin EL. Racial disparities in cardiovascular risk factors among diagnosed hypertensive subjects. J Am Soc Hypertens. 2011;5:239–248. [DOI] [PubMed] [Google Scholar]
- 11. Wright JD, Hughes JP, Ostchega Y, et al. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001–2008. Natl Health Stat Rep. 2011;35:1–22, 24. [PubMed] [Google Scholar]
- 12. Basile J, Neutel J. Overcoming clinical inertia to achieve blood pressure goals: the role of fixed‐dose combination therapy. Ther Adv Cardiovasc Dis. 2010;4:119–127. [DOI] [PubMed] [Google Scholar]
- 13. Nesbitt SD. Environmental, societal and genetic contributions to hypertension in African Americans. Curr Cardiovasc Risk Rep. 2008;2:181–186. [Google Scholar]
- 14. Flack JM, Sica DA, Bakris G, et al. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56:780–800. [DOI] [PubMed] [Google Scholar]
- 15. Nesbitt SD. Management of hypertension in African Americans. US Cardiol. 2009;6:59–62. [Google Scholar]
- 16. Nesbitt SD. Overcoming therapeutic inertia in patients with hypertension. Postgrad Med. 2010;122:118–124. [DOI] [PubMed] [Google Scholar]
- 17. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 18. Douglas JG, Bakris GL, Epstein M, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163:525–541. [DOI] [PubMed] [Google Scholar]
- 19. Flack JM, Sica DA. Therapeutic considerations in the African‐American patient with hypertension: considerations with calcium channel blocker therapy. J Clin Hypertens (Greenwich). 2005;7(4 Suppl 1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention . Vital Signs: prevalence, treatment, and control of hypertension – United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:103–108. [PubMed] [Google Scholar]
- 21. Gosse P, Lasserre R, Minifie C, et al. Blood pressure surge on rising. J Hypertens. 2004;22:1113–1118. [DOI] [PubMed] [Google Scholar]


