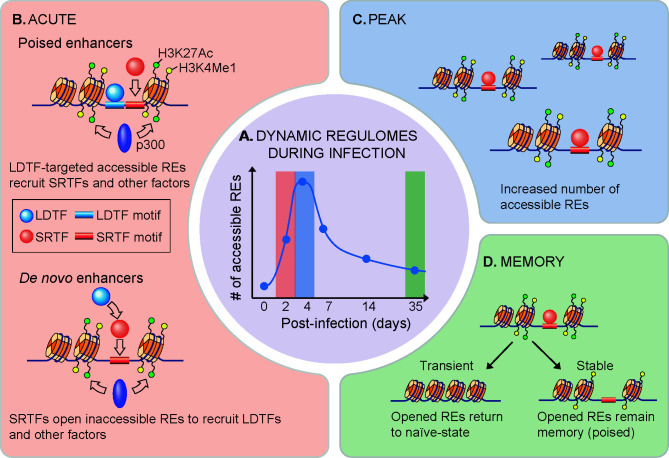
Figure 1.
Dynamics of NK cell regulomes during infection. (A) Dynamic regulomes during infection. Innate immune response occurs along with changes in gene expression as well as chromatin accessibility. (B) High-magnitude gene upregulation during NK cell activation relies on recruitment of signal-regulated transcription factors (SRTFs) to poised enhancers that are developmentally acquired in a lineage-determining transcription factors (LDTFs) manner for chromatin remodeling (top) (72). High-magnitude gene induction also forms de novo enhancers through a process involving sequence-specific binding of SRTFs to inaccessible chromatin regions, followed by LDTF recruitment and enhancer activation (bottom) (72). (C) Formation of new accessible sites rapidly occurs in vivo upon mouse cytomegalovirus or Toxoplasma gondii infection until a peak of the response is reached (69). (D) At the end of viral infection, majority of these rapidly opened chromatins return to resting state, while part of them undergo stable epigenetic poising that maintains NK cell adaptive-like or memory phenotype (69, 70).