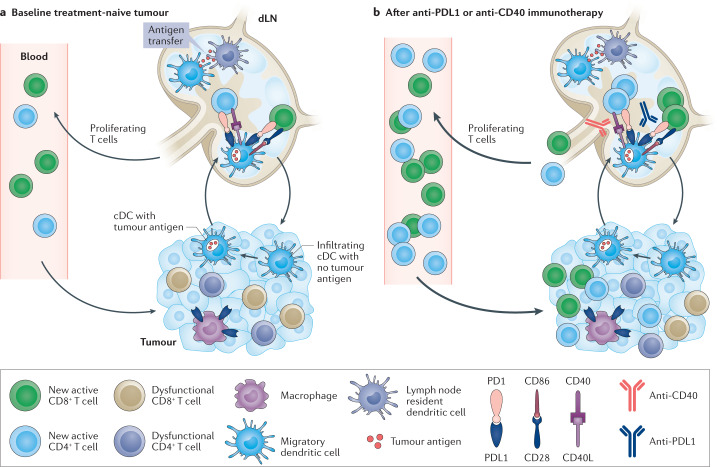
Fig. 2. Systemic immune responses in cancer immunotherapy.
Effective responses to immunotherapy drive de novo peripheral immune responses. Schematic illustrating how functional antitumour responses are reliant on immune dynamics outside the tumour microenvironment (TME). a | At baseline, conventional dendritic cells (cDCs) in the TME take up tumour antigen and travel to the draining lymph node (dLN), where they can then transfer antigen to resident cDCs through the formation of direct synapses. T cells in the TME reach states of terminal exhaustion due to chronic stimulation, the harsh environment and immunosuppressive cues. Dysfunctional intratumoural T cells accumulate structurally damaged mitochondria, and upregulate CD103 and CD38 coinciding with irreversible epigenetic remodelling. Thus, effective antitumour responses driven by therapy must rely on another source of functional effector T cells. b | Immunotherapeutic intervention through PD1 and PDL1 checkpoint blockade increases the interaction between cDCs and naive T cells in the dLN, and, alongside CD28 co-stimulation, facilitates the priming and rapid expansion of new T cell clones with new antigen specificities. Checkpoint blockade also leads to the proliferation of existing T cell clones in circulation. These expanding peripheral T cells ultimately infiltrate the TME, and express markers indicative of antigen-specific activation and demonstrate functional cytotoxicity. Productive de novo immune responses can also be achieved through CD40 agonism, which can drive cDC activation in settings resistant to checkpoint blockade and initiate these new T cell responses to replace exhausted intratumoural clones.