FIGURE 1.
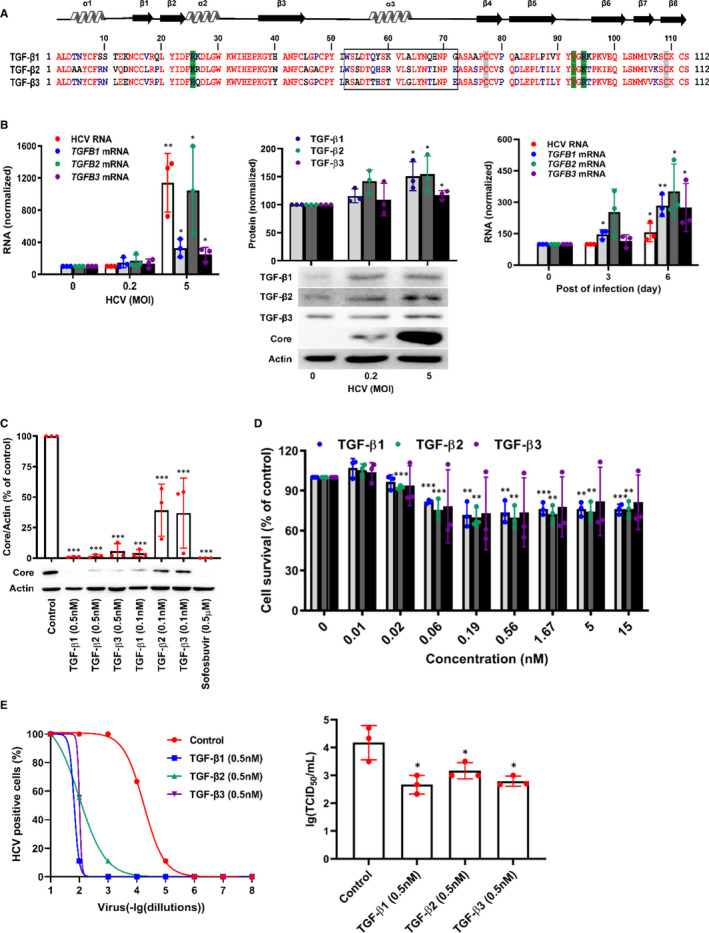
TGF‐β isoforms inhibit HCV propagation in HCVcc system. A, Sequence alignment of C‐terminal domains of mature human TGF‐β isoforms. Green shading represented the amino acid residues contacting with TβRII; grey shading represented the cysteine residues that formed the inter‐chain disulphide; open box designated the residues of α‐helix 3 in TGF‐β isoforms; the homology sequence of TGF‐β isoforms was indicated in red text. B, Huh7.5 cells were treated with HCV at different MOI for 72 h or at MOI = 0.2 for 0, 3 days or 6 days, and then TGF‐β isoforms mRNA or protein were quantified by qRT‐PCR or WB analysis. Huh7.5 cells were infected with HCV for 72 h in the presence of TGF‐β isoforms or sofosbuvir. Intracellular proteins were extracted and detected by WB (C) and the cytotoxicity without HCV infection was identified using an MTT assay (D). E, Huh7.5 cells were incubated with HCV viral stock at 10‐fold dilutions ranged from 10−1 to 10−8, and simultaneously treated with 0.5 nmol/L of TGF‐β1, TGF‐β2 and TGF‐β3. At 72 h, HCV‐positive wells were examined using a microscope after immunostaining against HCV core protein (left). Huh7.5 cells were inoculated with HCV viral stock and 0.5 nmol/L of TGF‐β1, TGF‐β2 or TGF‐β3 for 24 h. The newly released infectious virus particles were collected at 48 h and then quantified by TCID50 assay (right). Data represent the mean ± SD of three independent experiments. ANOVA analysis followed by the Student's t test method was used. *P < 0.05, **P < 0.01, ***P < 0.001 vs the control group
