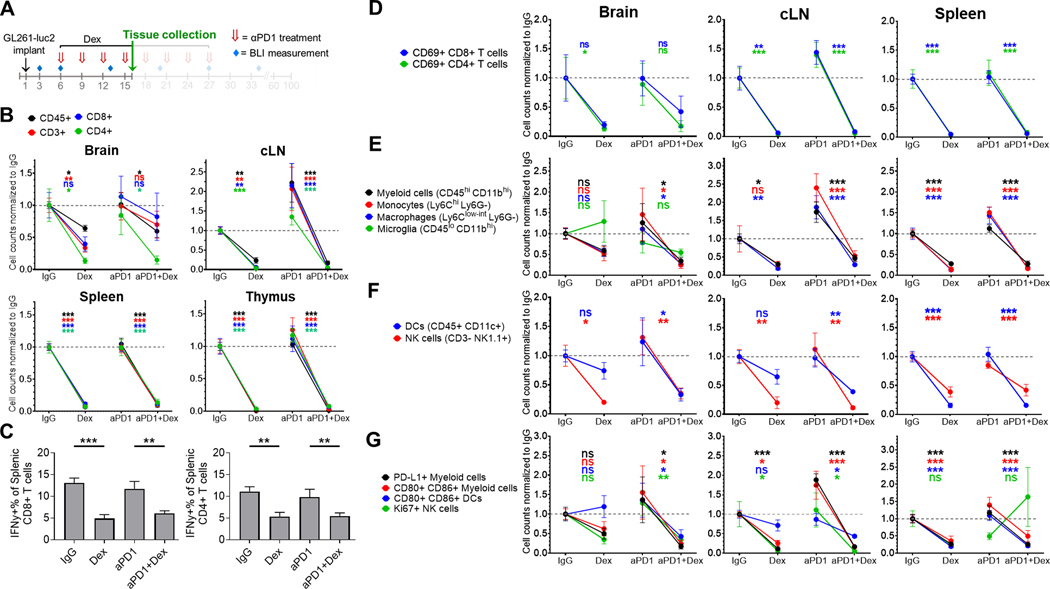
Figure 3. Concurrent dexamethasone negatively affects intratumoral and systemic adaptive and innate immune cell populations in the GL261-luc2 glioblastoma mouse model.
(A) Experimental schema. Tissue was collected at day 16 of a dose-intensive regimen of anti-PD-1, in which anti-PD-1 (αPD1) was administered IP beginning on day 6 (500 μg loading dose) followed by 3 additional doses (250 μg) at 3-day intervals, with dexamethasone (10 mg/kg) administered IP on days 6–16. Tissue (n=4–8/group, derived from two experiments) was harvested on day 16 and analyzed by flow cytometry. Immune cell counts were evaluated by multiple linear regression, normalized to the corresponding IgG control group’s mean count (displayed as dashed gray line), and displayed as mean ± SE. (B) Differences in CD45+ leukocytes and CD45+ CD3+ lymphocytes, including CD4+ and CD8+ T cells between treatment groups. (C) Percentage of splenic IFNγ+ CD4+ and CD8+ lymphocytes by treatment group. (D) Change in the number of early activated CD69+ T cells by site for each treatment group. Additionally, differences between treatment groups in innate immune cells including (E) myeloid cells (CD45hi CD11bhi), macrophages (Ly6Clo-int Ly6G-), monocytes (Ly6Chi Ly6G-), and microglia (in the brain, CD45lo CD11bhi), (F) dendritic cells (DCs; CD45+ CD11c+) and NK cells (CD45+ CD3- NK1.1+); as well as (G) activated (CD80+ CD86+) myeloid cells and DCs, PD-L1+ myeloid cells, and Ki67+ NK cells were analyzed.
cLN, cervical lymph node; Dex, dexamethasone; ns, not significant, p≥0.05; *p<0.05; **p<0.01; ***p<0.001