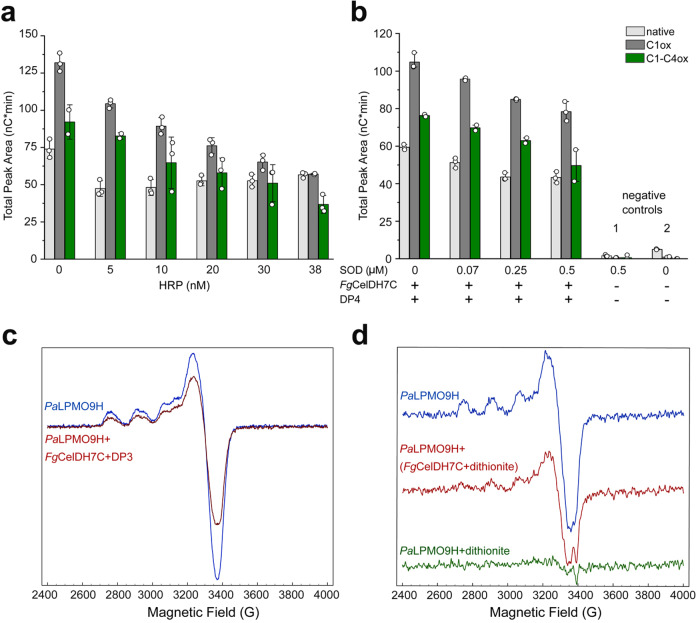
Fig. 5. Mechanistic insights into the activation of LPMO by FgCelDH7C.
a, b Effect of horseradish peroxidase (HRP) and superoxide dismutase (SOD) on the interplay between PaLPMO9H (4 μM) and FgCelDH7C (0.41 μM) in Avicel degradation based on the total area of native, C1 oxidised (C1 ox) and double C1-C4 oxidised (C1-C4ox) cello-oligosaccharides as analysed by HPAEC-PAD. Controls prepared in the absence of FgCelDH7C (1) or in the absence of both SOD and FgCelDH7C (2). The assays were performed for 18 h at 35 °C and terminated using NaOH (0.1 M) prior to the HPAEC-PAD analyses. The total peak area (white circles, n = 3 independent experiments) are shown and the bar plots display the means with standard deviations. c X-band Electron Paramagnetic Resonance (EPR) spectra of PaLPMO9H−Cu(II) (100 μM) in the presence of cellotriose (DP3, 1 mM) before (blue line) and after (red line) addition of FgCelDH7C (AA7). d X-band EPR spectra of PaLPMO9H−Cu(II) (20 μM) in buffer (blue line), in the presence of FgCelDH7C (AA7) pre-reduced with dithionite (red line), or directly fully reduced with dithionite (10 eq., green line). All EPR solutions and experiments were performed under anaerobic conditions. Samples were in 50 mM NaOAc, pH 5.2 and spectra were recorded at 50 K with a 4 mW microwave power and a 30 Gauss modulation amplitude. The data in (c) and (d) are based on a single experiment (n = 1). Source data for the a and b panels are provided as a Source Data file.