Fig. 3.
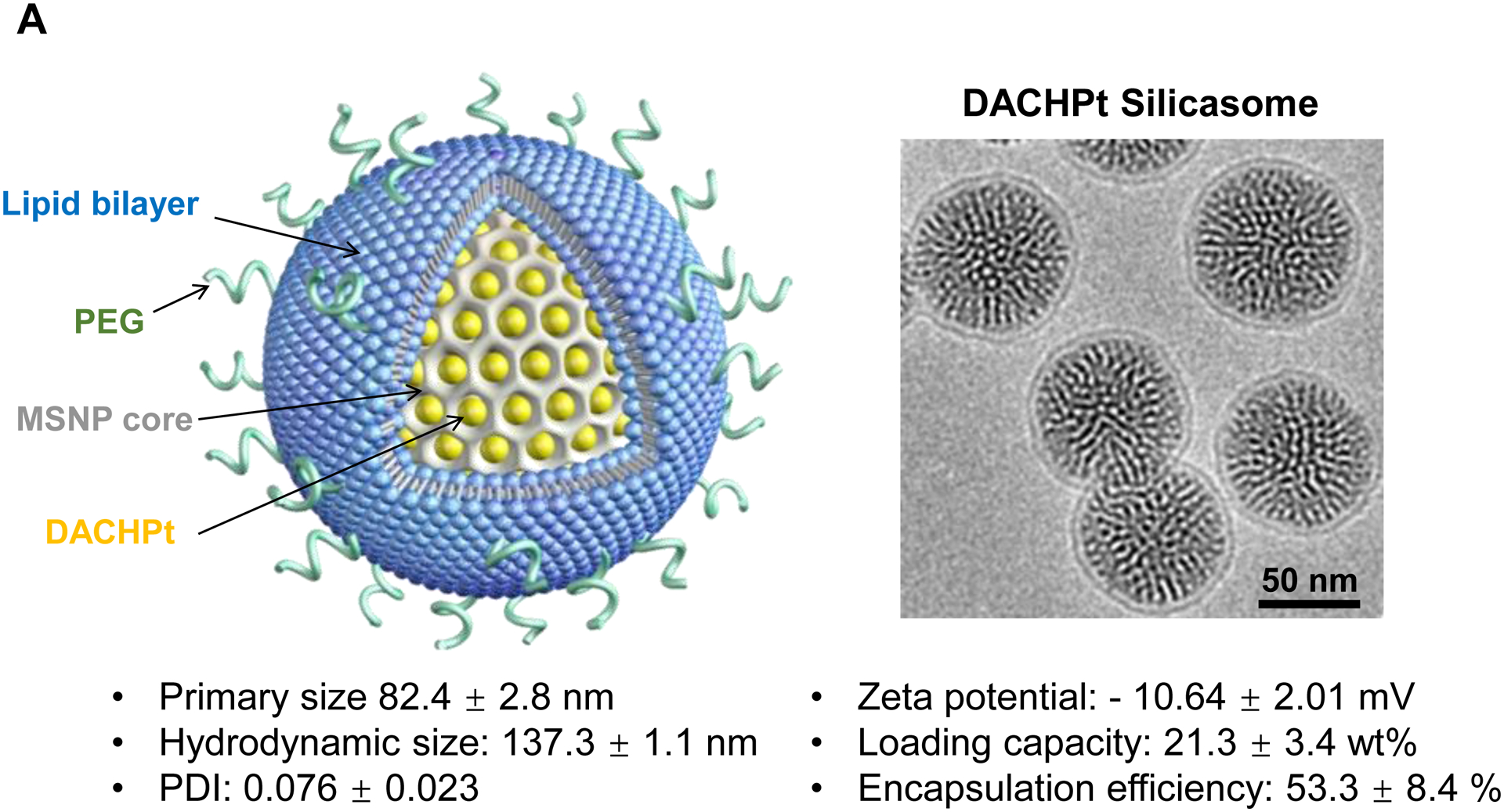
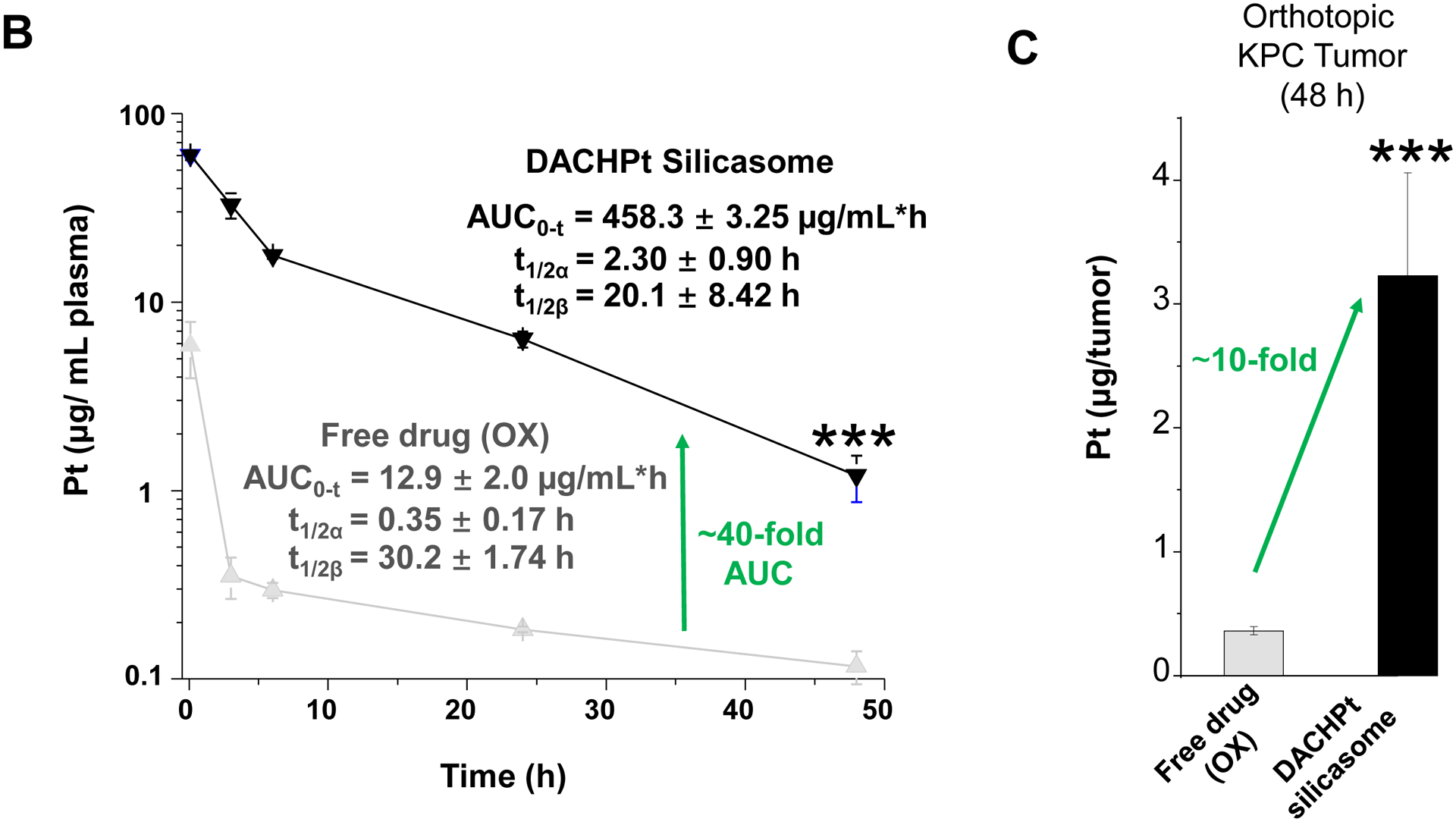
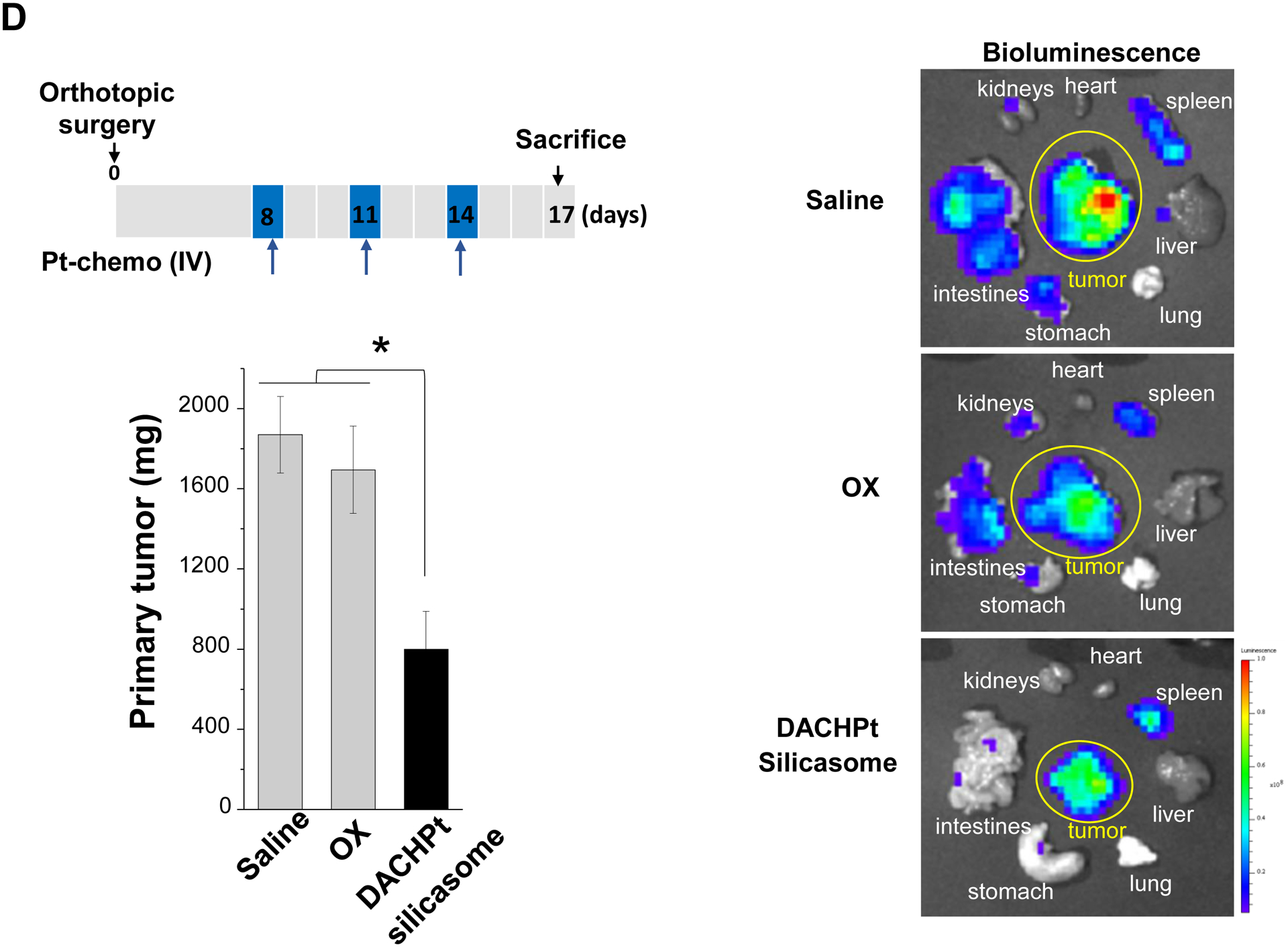
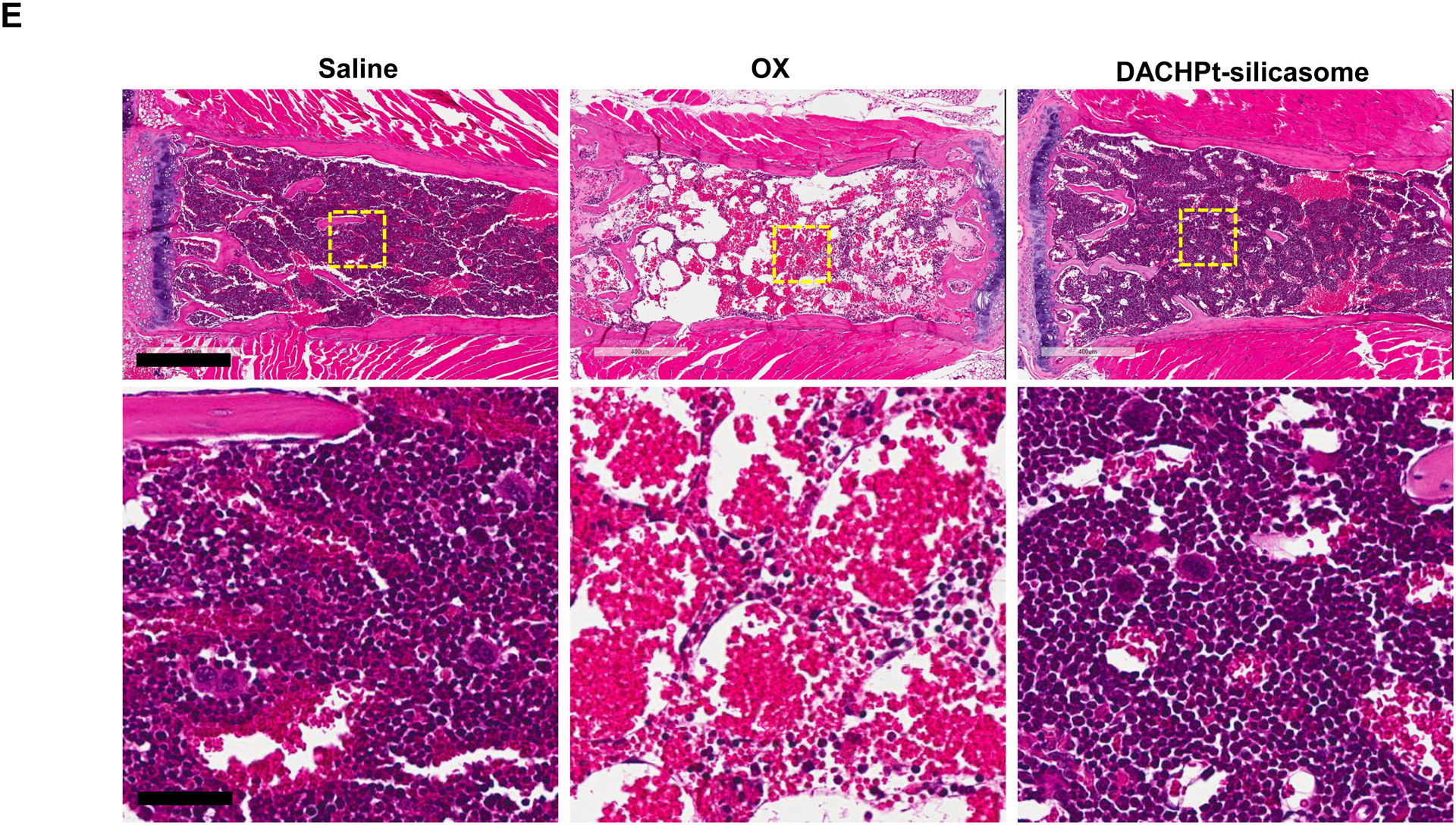
The DACHPt silicasome improves the PK, biodistribution and anti-cancer efficacy over the free drug in a KPC-derived orthotopic model. The encapsulated delivery also improves drug safety. (A) Before animal experimentation, the DACHPt silicasomes were fully characterized, including by cryoEM visualization. The physicochemical properties are summarized. (B-C) PK profile in healthy mice (B) and Pt drug content in orthotopic KPC tumor (C) after the animals received a single IV injection of free oxaliplatin or DACHPt silicasome at identical Pt dose, i.e. 4.95 mg/kg (n = 3). PK parameters were calculated by PKSolver software. Pt content was quantified by ICP-MS. Data represent mean ± SD; ***, p < 0.001 (two-tailed Student’s t-test). (D) Comparative efficacy testing of DACHPt silicasome vs free oxaliplatin in the orthotopic KPC model in B6129SF1/J mice. KPC-luc tumor-bearing mice received the free drug or DACHPt silicasomes 8 days after initial tumor implantation. A total of 3 IV administrations were performed. Saline was used as a negative control. In addition to assessing primary tumor size by weight, tumor size and metastases were also assessed by IVIS imaging as shown in the right-hand panel. Data represent mean ± SEM; *, p < 0.05 (one-way ANOVA followed by a Tukey’s test). (E) Histological analysis of bone marrow by H&E staining in the efficacy experiment in (D). Bar in upper panel is 400 μm and in lower panel is 50 μm. Additional histological analysis to show treatment safety in various organs are shown online (Fig. S7).
