Fig. 3 |. Model for cellular senescence in joint tissue and potential treatments.
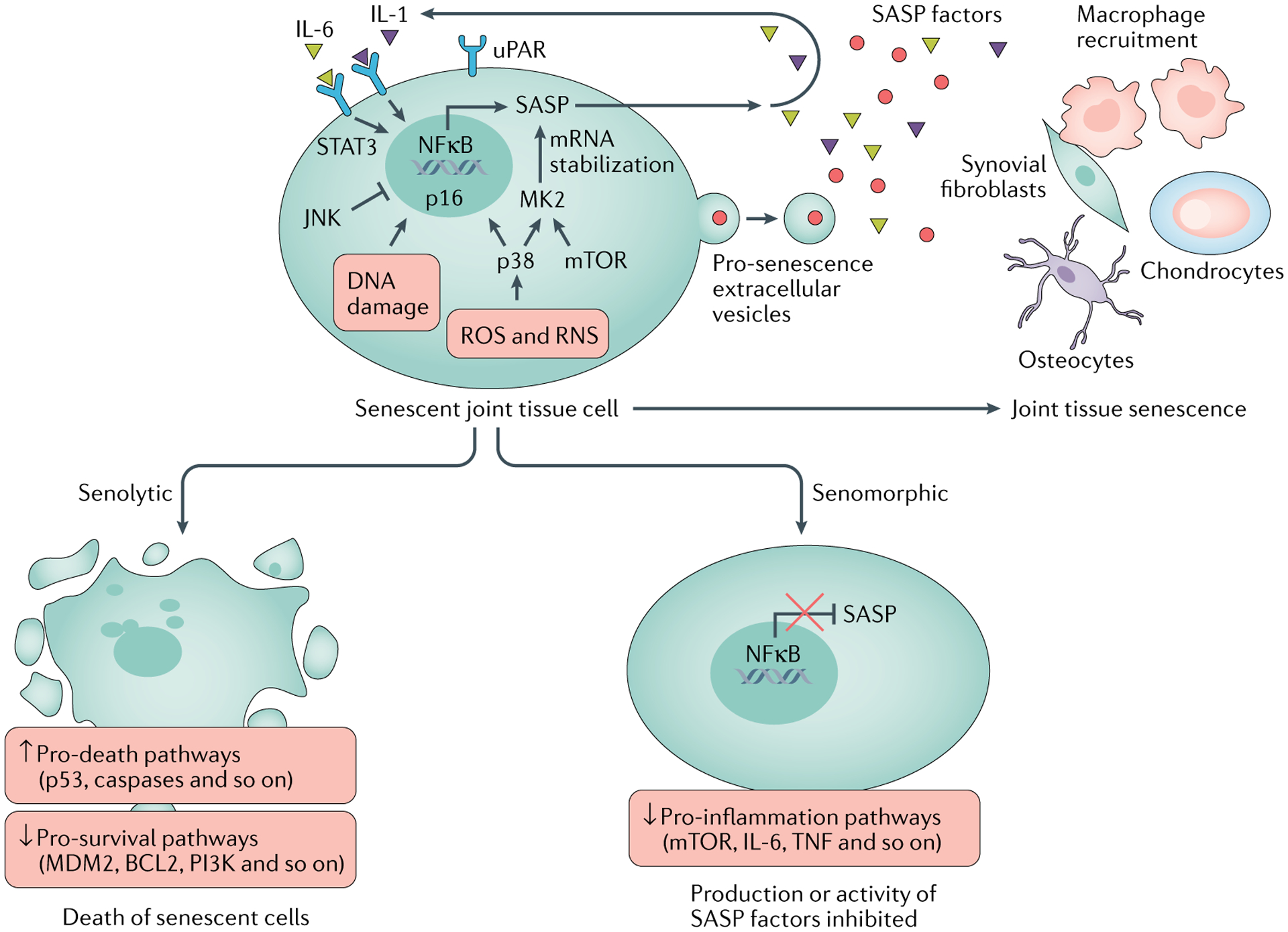
Cytokines such as IL-6 promote senescence via the transcription factor STAT3, and IL-1 can induce NFκB-driven expression of genes encoding senescence-associated secretory phenotype (SASP) factors. Senescent joint cells are characterized by increased oxidative stress (owing to the generation of reactive oxidative species (ROS) and reactive nitrogen species (RNS)), DNA damage, increased expression of urokinase-type plasminogen activator surface receptor (uPAR), and upregulation of stress proteins such as p38, c-Jun N-terminal kinase (JNK) and mTOR. p38 induces senescence and the expression of p16, while JNK negatively regulates senescence in cells in joint tissue. mTOR and p38 promote the SASP by upregulating the translation of (mTOR) and phosphorylating (p38) MK2 (also known as MAPKAPK2), which stabilizes mRNA transcripts encoding SASP factors. SASP factors (including IL-1 and IL-6) and senescence-inducing extracellular vesicles are secreted by these cells into the extracellular matrix, promoting macrophage recruitment to, and driving further senescence in, the surrounding joint tissue. Senolytic drugs aim to prevent senescence-associated disease by inducing apoptosis specifically in senescent cells via the upregulation of p53, caspases and other proteins in death-associated pathways, while repressing pathways associated with cell survival (for example, pathways involving MDM2, BCL2 and PI3K). Senomorphic drugs do not kill senescent cells, but repress the SASP by inhibiting the activity of proteins related to inflammation, such as mTOR, or by directly inhibiting the activity or production of SASP factors such as IL-6 and TNF.
