Fig. 2.
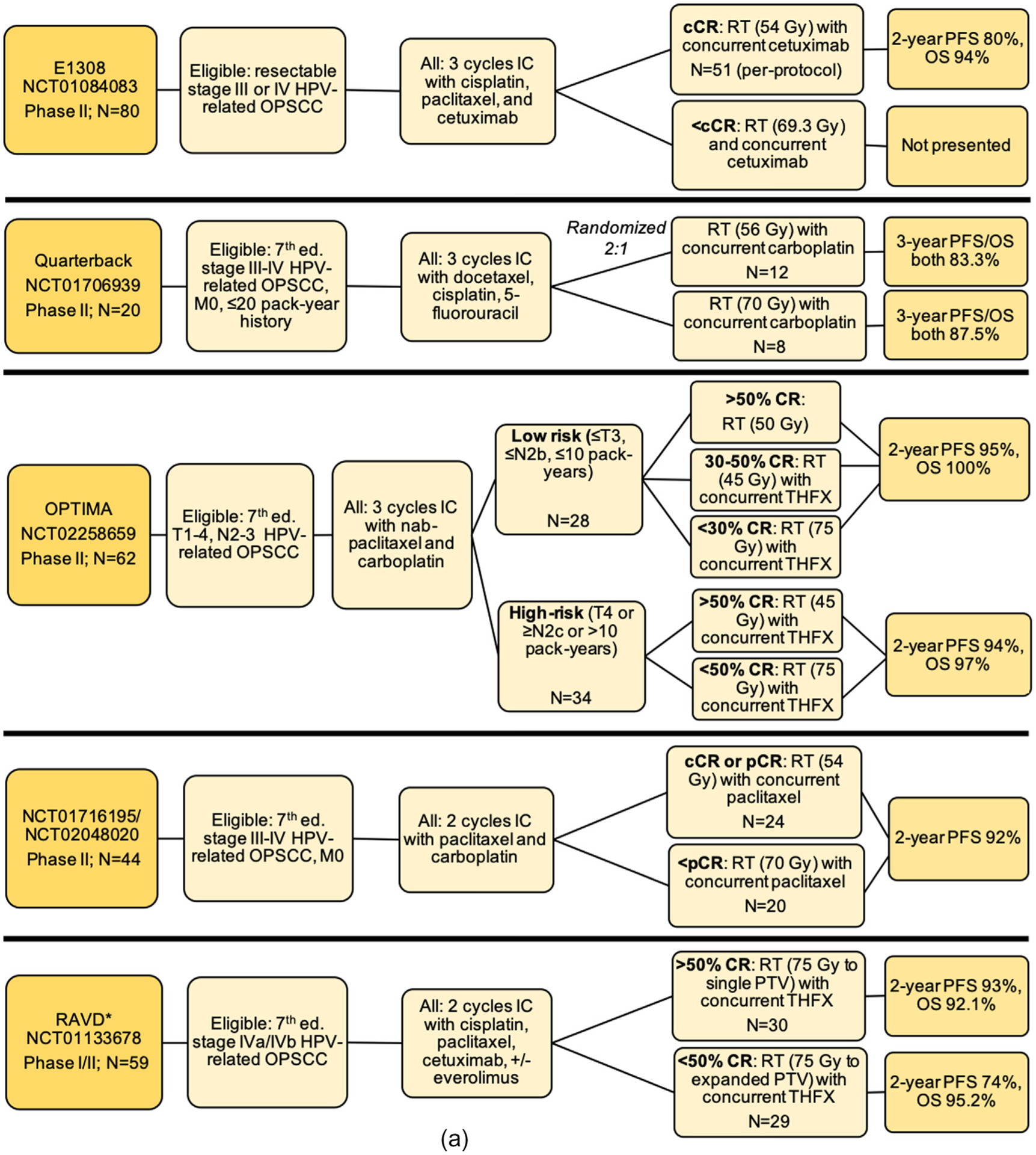
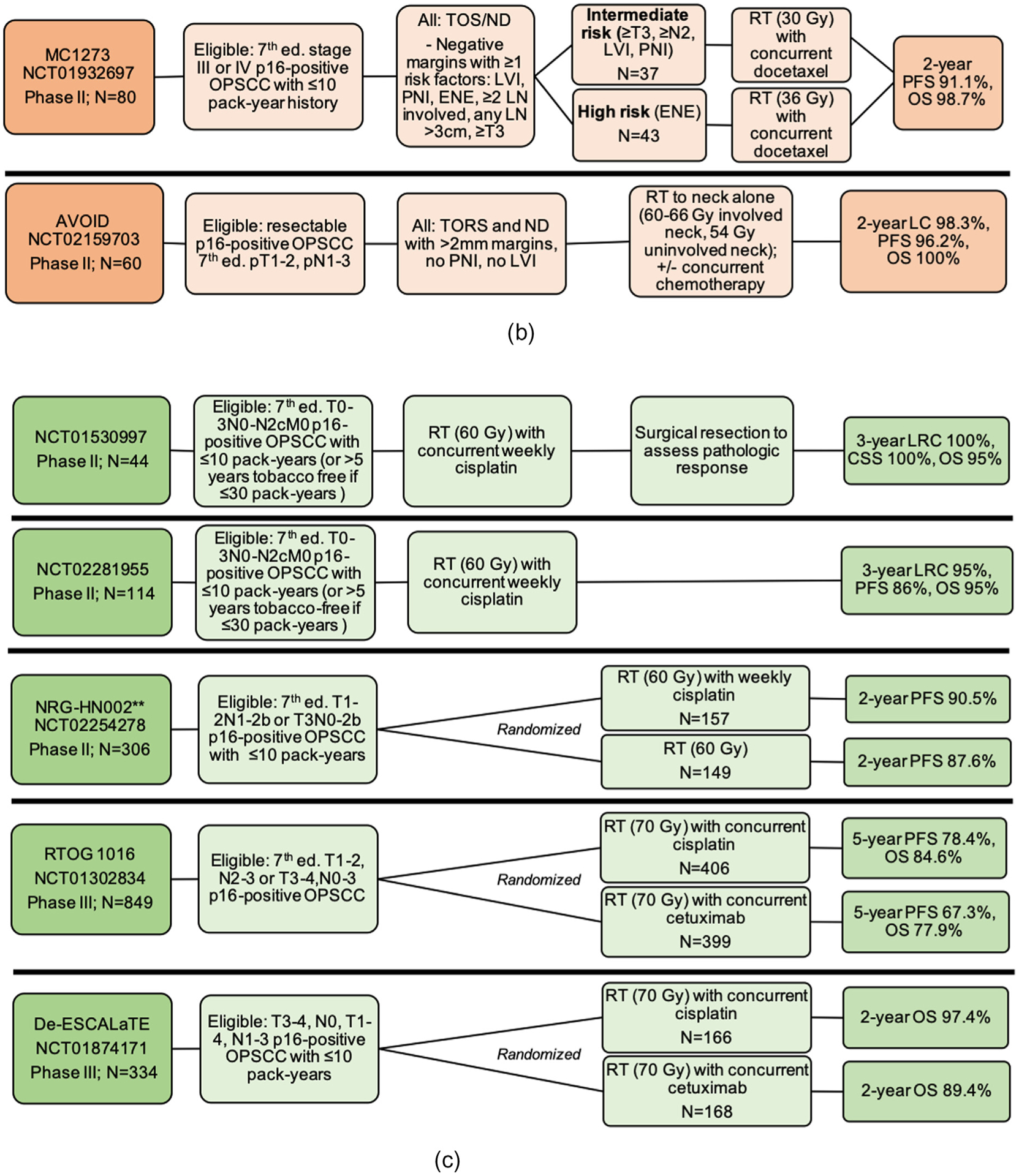
Schemas for completed treatment deintensification trials for HPV-associated OPSCC including (a) trials testing de-escalation based on induction response; (b) trials testing de-escalation of adjuvant therapy after surgery; (c) trials testing primary chemoradiation de-escalation, Notes: *RAVD enrolled stage IVa/IVb HNSCC inclusive of all primary sites and without regard to HPV status. However, a subset analysis of HPV-OPSCC patients was performed. Of 94 total enrolled patients, 71 patients had an oropharynx primary site, out of whom 59 patients had positive HPV-status. The data presented here are specific to the HPV-associated OPSCC patients only and is not inclusive of the larger study population. **NRG-HN002: results have been presented and published as an abstract, Abbreviations: Ed. = edition; OPSCC = oropharyngeal squamous cell carcinoma; IC = induction chemotherapy; RT = radiation therapy; CRT = chemoradiotherapy; CR = clinical response; cCR = complete clinical response; pCR = partial clinical response; OS = overall survival; PFS = progression free survival; LRC = locoregional control; LC = local control; CSS = cause-specific survival; LVI = lymphovascular invasion; PNI = perineural invasion; ENE = extranodal extension; LN = lymph node; TOS = transoral surgery; TORS = transoral robotic surgery; ND = neck dissection; THFX: chemotherapy regimen consisting of paclitaxel, 5-fluorouracil, and hydroxyurea; PTV = primary target volume.
