Abstract
Background:
Programmed cell death ligand 1 (PD-L1), which is highly expressed in a variety of malignant tumors, is closely related to clinicopathological features and prognosis. However, there are few studies on the potential effects of PD-L1 on thyroid carcinoma, the incidence of which has shown an upward trend worldwide. This study aimed to explore the association between PD-L1 expression and clinicopathological features and prognosis of thyroid cancer.
Methods:
An elaborate retrieval was performed using Medline, PubMed, Cochrane Library, EMBASE, Web of Science, WanFang databases, and China National Knowledge Infrastructure to determine the association between PD-L1 expression and disease-free survival (DFS), overall survival (OS), and clinicopathological features in patients with thyroid cancer. Study selection, data extraction, risk assessment, and data synthesis were performed independently by 2 reviewers. In this meta-analysis, RevMan 5.3 and Stata 15.1 were used for bias risk assessment and data synthesis.
Results:
After a detailed search, 2546 cases reported in 13 articles were included in this meta-analysis. The outcomes revealed that high expression of PD-L1 in patients with thyroid cancer was associated with poor DFS (hazard ratio [HR] = 3.37, 95% confidence interval [CI] 2.54–4.48, P < .00001) and OS (HR = 2.52, 95% CI: 1.20–5.32, P = .01). High PD-L1 expression was associated with tumor size ≥2 cm, tumor recurrence, extrathyroidal extension, concurrent thyroiditis, unifocal tumor, and absence of psammoma body (P < .05). Subgroup analysis showed that positive expression of PD-L1 was related to poor prognosis for DFS of non-medullary thyroid carcinoma, and the overexpression of PD-L1 in differentiated thyroid carcinoma (DTC) was related to tumor recurrence, concurrent thyroiditis, extrathyroidal extension, unifocal DTC, late stage DTC, and BRAFV600E mutation in DTC.
Conclusion:
PD-L1 is a significant predictor of prognosis and malignancy of thyroid cancer (especially DTC), and PD-L1 inhibitors may be a promising therapeutic option for refractory thyroid cancer in the future.
Keywords: clinicopathological features, disease-free survival, meta-analysis, overall survival, programmed cell death ligand 1, prognosis, thyroid cancer
1. Introduction
For nearly 30 years, there has been an upward trend in the incidence of thyroid cancer in different parts of the world.[1] The global incidence of thyroid cancer among women was approximately 5.1% in 2018, ranking fifth in the global cancer incidence among women.[2] Thyroid cancer includes differentiated thyroid carcinoma (DTC), poorly DTC (PDTC), anaplastic thyroid carcinoma (ATC), and medullary thyroid carcinoma (MTC). MTC originates from parafollicular thyroid cells, whereas all other thyroid cancers originate from thyroid follicular epithelial cells. Since ionizing radiation has been used in medical applications for the diagnosis and treatment of diseases, the doses to which patients have been exposed are increasingly higher, and an increase in the mean out-of-field dose is related to field size, depth, energy, etc. This has led to an increase in the incidence of cancers such as thyroid cancer.[3,4] MTC occurs in 1% to 2% of cases of thyroid cancers.[5] DTC is the most common type thyroid cancer, accounting for 90% of all thyroid cancers; papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) account for 80% and 10% of thyroid cancers, respectively.[6] The incidence of PDTC is 2% to 15%,[7] and its morphology and clinical behavior are usually between those of DTC and ATC. ATC is the most malignant and rare form of thyroid cancer (incidence, 1%).[5] The routine treatments for thyroid cancer include hormone suppression therapy, surgery, and radioiodine therapy. The 10-year survival rate of radioiodine-refractory patients is approximately 19%,[8] and the survival of patients with ATC is worse; moreover, 50%[9] of patients with ATC die after onset. Therefore, a new method to predict the prognosis and malignancy of thyroid cancer has become a hot topic in clinical research.
At present, programmed cell death ligand 1 (PD-L1) is used as a prognostic marker in melanoma, non-small cell lung carcinoma, breast carcinoma, and other cancers. PD-L1 is widely expressed in dendritic cells, T cells, B cells, macrophages, vascular endothelial cells, islet cells, and a variety of tumor cells.[10] Programmed cell death receptor 1 (PD-1), the receptor of PD-L1, is an essential immunosuppressive molecule that is expressed in a variety of immune cells including monocytes, regulatory T cells, CD4+ T cells, CD8+ T cells, B cells, natural killer cells, dendritic cells, and antigen presenting cells.[10,11] Under pathological conditions, tumor antigens stimulate PD-1 expression in immune cells, and PD-L1 of tumor cells binds to PD-1; this inhibits the function of the immune system and weakens the anti-tumor immune response of the body, leading to the immune escape of tumor and promoting the proliferation and development of tumor cells.[12] Numerous studies[13,14] have shown that PD-L1 expression in a variety of malignant tumors is closely related to the clinicopathological features and prognosis of cancers. The Food and Drug Administration has approved the use of anti-PD-1/PD-L1 monoclonal antibodies for the treatment of melanoma, non-small cell lung cancer, urothelial carcinoma, and other cancers.
The association between PD-L1 expression and thyroid cancer, as well as the clinical characteristics and prognosis of thyroid cancer, remains unclear. In this study, a meta-analysis was performed to determine the association between PD-L1 expression and thyroid cancer to provide clinicians with insights into the development of new drugs for the treatment of thyroid cancer. Relative to the meta-analysis conducted in 2018,[15] this meta-analysis assessed the association between PD-L1 expression and overall survival (OS); relative to the meta-analysis conducted in 2020,[16] the relationship between PD-L1 expression and tumor bipartition, stromal calcification, psammoma body, and capsular invasion were evaluated in this meta-analysis.
2. Materials and methods
2.1. Ethics statement
Because the data from this meta-analysis were based on previous studies, this study did not require ethical approval or patient consent.
2.2. Search strategy
Articles were systematically selected by 2 researchers (W-BY and D-PY) independently from EMBASE, Web of Science, Medline, PubMed, Cochrane Library, WanFang databases, and China National Knowledge Infrastructure from the date of establishment of the databases to May 2020. The keywords used were “programmed death-ligand 1” and “thyroid neoplasm” or their derivations and different combinations. Moreover, references to the relevant literature were carefully checked to include more potential articles.
2.3. Inclusion and exclusion criteria
2.3.1. Inclusion criteria for the meta-analysis
-
(1)
Subjects: Patients with thyroid cancer, regardless of age, sex, ethnicity, or nationality.
-
(2)
Research method: Positive expression of PD-L1 was set as the inclusion criteria for the experimental group, and negative expression of PD-L1 for the control group. The diagnostic method for thyroid cancer was pathology. The expression of PD-L1 in thyroid cancer was demonstrated by immunohistochemistry (IHC).
-
(3)
Outcome indicators: Disease-free survival (DFS) or OS.
-
(4)
Clinicopathological indicators: Age, sex, tumor size, recurrence, tumor–nodes–metastases (TNM) stage, extrathyroidal extension, capsular invasion, lymphovascular invasion, multifocality, concurrent thyroiditis, psammoma body, calcification, BRAFV600E, or laterality.
2.3.2. Exclusion criteria for the meta-analysis
-
(1)
The subject of the study was not human.
-
(2)
Without original data (such as reviews, meta-analyses, letters, case reports, meeting abstracts, expert consensus, or critical articles).
-
(3)
Duplicate literature and literature that did not contain relevant data (pathological characteristics and prognosis of thyroid cancer).
-
(4)
Low-quality articles: Newcastle–Ottawa Scale (NOS) score <4.
2.4. Literature screening and data extraction
All researchers received complete instructions for a systematic review. Two researchers (W-BY and D-PY) completed the literature study independently in accordance with established inclusion and exclusion procedure. The differences in results of the 2 researchers were resolved through joint discussion of literature. In cases of disagreement, a third researcher (D-WL) solved the problem. The following data were extracted: basic information including first author, country, publication year, sample size, median age, positive rate of PD-L1, cutoff value of PD-L1, and positive judgment; clinicopathological features including age at diagnosis (≥45 or <45 years), sex (male or female), tumor size (≥2 or <2 cm), recurrence (positive or negative), TNM stage (stages III–IV or I–II), extrathyroidal extension (positive or negative), capsular invasion (positive or negative), lymphovascular invasion (positive or negative), multifocality (multifocal or solitary), thyroiditis (present or absent), psammoma body (present or absent), calcification (present or absent), BRAFV600E (mutated or wild type) and laterality (unilateral or bilateral distribution), and number of positive and negative clinical signs of PD-L1 induced to investigate the relationship between PD-L1 and clinical endpoints; and outcome indicators including DFS and OS, and hazard ratio (HR) with the corresponding 95% confidence interval (CI) were extracted to evaluate the relationship between PD-L1 expression and DFS and OS. When relevant data were unavailable, we obtained the information from the first or corresponding authors.
2.5. Quality assessment
Two researchers (W-BY and D-PY) independently evaluated the quality of the selected articles using the NOS. Differences were resolved by consultation or discussion with a third researcher (D-WL). The NOS consists of 8 projects divided into 3 main sections: selection of random checks, comparability of groups, and identification of related results. The full score of NOS was 9 points: 7 or more points were classified as high-quality literature, 4 to 6 as medium-quality literature, and 0 to 3 as low-quality literature.
2.6. Statistical analysis
Statistical analysis was performed using RevMan 5.3 (Copenhagen: The Nordic Cochrane Centre, 2014) and STATA (StataCorp, College Station, TX, USA) analysis software, and the association between PD-L1 expression and DFS, OS, and clinicopathological features was evaluated by calculating the pooled HRs or odds ratios (ORs) with 95% CIs. Subgroup analysis was implemented based on the type of thyroid cancer and the source of countries. Heterogeneity across studies was evaluated using the Q-test, and the results were expressed as I2 and P values. If P ≤ .10 and I2 ≥ 50%, there was apparent heterogeneity among the studies. In this case, a random-effects model was applied, and sensitivity analysis and Galbraith graph were used to determine the heterogeneous sources; otherwise, a fixed-effects model was employed. Publication bias was evaluated by adopting the funnel plot, Egger test, and Begg test, and the final results were subjected to Egger test if they were different. Differences were considered statistically significant at P < .05.
3. Results
3.1. Literature search and study selection
The flowchart in Fig. 1 shows the article selection process by the 2 independent researchers. In the first group, 716 articles were extracted, 564 of which were repetitive or unqualified and were excluded. During abstract screening, we found that 123 articles included non-human experiments, evaluations, meta-analyses, letters, case reports, or conference abstracts and were excluded; 16 articles were excluded due to poor quality or insufficient data. The remaining 13 articles[17–29] were included in our analysis.
Figure 1.
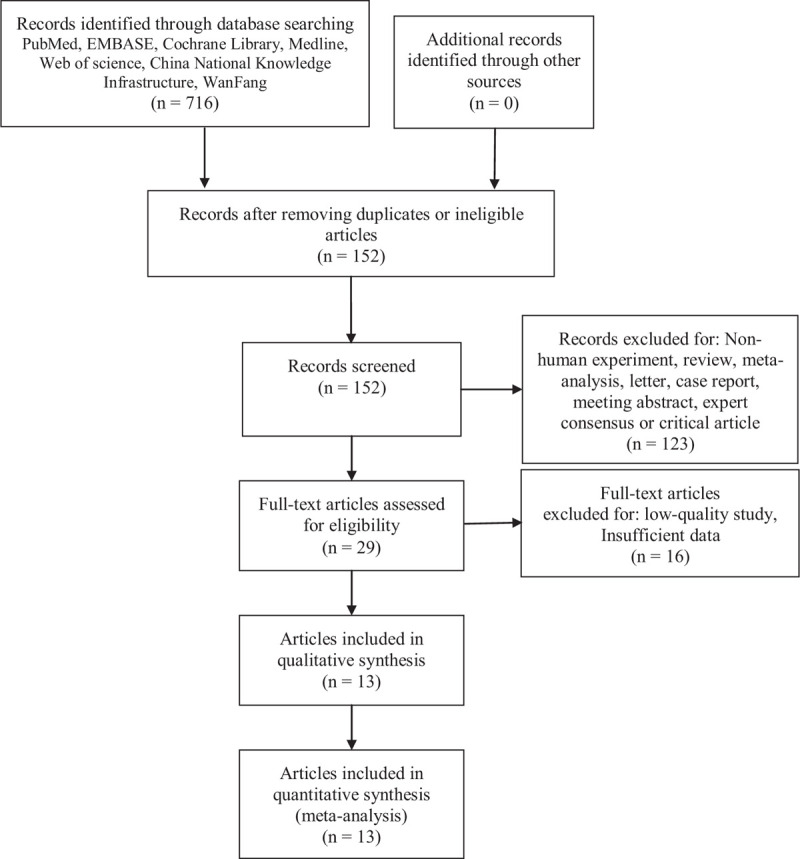
Flow diagram of article selection.
3.2. Tumor characteristics in studies
A total of 13[17–29] relevant articles (include 2546 cases) were extracted through detailed exclusion and inclusion criteria. However, these articles included 17 studies in total because Chowdhury et al[24] included 2 studies according to the cut-off value for PD-L1 in the cytoplasm and membrane and Aghajani et al[18] included 4 studies according to the cut-off value for PD-L1 in the serum and plasma. Among the 17 studies, 8 recorded the survival outcomes of patients with thyroid cancer,[17,18,22–26,29] and all studies recorded pathological characteristics.[17–29] The tumor types in 8 articles were DTC,[17,18,20,21,24,25,27,29] 2 were PDTC or ATC,[23,26] 2 were MTC,[22,28] and 1 was PTC + FTC + PDTC + ATC.[19]Table 1 summarizes the features of the included studies.
Table 1.
General information of included studies.
| First author (year) | Country | Sample size | Age | Tumor type | Detection method | No. of PD-L1 positive (%) | Cut-off value of PD-L1 | Outcome | NOS |
| Aghajani (2017)[17] | Australia | 75 | 51 (24–83) | PTC | IHC | 50 (66.7%) | Allred scores >1 | DFS | 7 |
| Shi (2017)[27] | China | 260 | 46.6 (14–80) | PTC | IHC | 136 (52.3%) | Staining score ≥6 | RFS | 7 |
| Shi (2019)[28] | China | 201 | 49 (12–80) | MTC | IHC | 29 (14.4%) | CPS ≥1 | SRFS | 6 |
| Ahn (2017)[19] | Korea | 407 | 43.8 (16–81) | PTC, FTC, PDTC, ATC | IHC | 27 (6.6%) | ≥1% threshold | DFS | 6 |
| Chintakuntlawar (2017)[23] | USA | 16 | 58 (37–83) | ATC | IHC | 6 (37.5%) | Tumor cells >33% | DFS, OS | 8 |
| Zhou (2019)[29] | China | 85 | NM | FTC | IHC | 57 (67.1%) | NM | NM | 5 |
| Cunha (2013)[25] | Brazil | 293 | 43.6 | DTC | IHC | 244 (83.3%) | Allred scores ≥1 | DFS | 6 |
| Chowdhury1∗ (2016)[24] | Canada | 185 | 45 (18–85) | PTC | IHC | 123 (66.5%) | Cytoplasm score ≥4.5 | DFS | 7 |
| Chowdhury2∗ (2016)[24] | Canada | 185 | 45 (18–85) | PTC | IHC | 74 (40.0%) | Plasma membrane score ≥2.1 | DFS | 7 |
| Aghajani1∗ (2019)[18] | Australia | 101 | 47.0 (20–80) | PTC | IHC | 60 (59.4%) | Serum sPD-L1 ≥0.37 ng/mL | NM | 7 |
| Aghajani2∗ (2019)[18] | Australia | 101 | 47.0 (20–80) | PTC | IHC | 61 (60.4%) | Plasma sPD-L1 ≥0.19 ng/mL | DFS | 7 |
| Aghajani3∗ (2019)[18] | Australia | 101 | 47.0 (20–80) | PTC | IHC | 49 (48.5%) | Serum sPD-L1 ≥0.48 ng/mL | NM | 7 |
| Aghajani4∗ (2019)[18] | Australia | 101 | 47.0 (20–80) | PTC | IHC | 61 (60.4%) | Plasma sPD-L1 ≥0.21 ng/mL | NM | 7 |
| Rosenbaum (2018)[26] | USA | 28 | 64.9 (14–86) | PDTC | IHC | 7 (25.0%) | H-score threshold ≥5 | DFS, OS | 6 |
| Bi (2019)[22] | China | 87 | 47 (21–73) | MTC | IHC | 19 (21.8%) | >1% threshold | DFS, OS | 6 |
| Bai (2017)[21] | China | 126 | NM | PTC | IHC | 67 (53.2%) | Diffuse cytoplasmic staining | NM | 6 |
| Bai (2018)[20] | China | 110 | NM | PTC | IHC | 51 (46%) | Diffuse cytoplasmic staining | NM | 6 |
ATC = anaplastic thyroid carcinoma, DTC = differentiated thyroid carcinoma, FTC = follicular thyroid carcinoma, IHC = immunohistochemistry, MTC = medullary thyroid carcinoma, NM = not-mentioned, PDTC = poorly differentiated thyroid carcinoma, PTC = papillary thyroid cancer, RFS = recurrence-free survival, SRFS = structural recurrence-free survival.
Chowdhury 2016[24] includes 2 studies (Chowdhury1 2016, Chowdhury2 2016) according to the cut-off value for PD-L1 in the cytoplasm and membrane, and Aghajani 2019[18] includes 4 (Aghajani1 2019, Aghajani2 2019, Aghajani3 2019, Aghajani4 2019) studies according to the cut-off value for PD-L1 in the serum and plasma.
3.3. Quality assessment
According to the NOS, the studies were generally of medium quality, with a mean NOS score of 6.5 (range, 5–8), the quality score of 5 articles[17,18,23,24,27] (9 studies) was 7 to 8, which were considered high quality, and that of 8 articles[19–22,25,26,28,29] (8 studies) was 5 to 6, which were considered medium quality. The final quality assessment of the included studies is presented in Table 1.
3.4. PD-L1 expression can be used as a biomarker to predict DFS
Seven articles[17,18,22–24,26,27] (8 studies) provided HRs and 95% CIs of DFS in thyroid cancer. As shown in Fig. 2, no obvious heterogeneity was observed in these studies (I2 = 4%, P = .40), and the fixed-effects model was implemented. The results revealed that positive PD-L1 expression was related to a decrease in DFS in patients with thyroid cancer (HR = 3.37, 95% CI: 2.54–4.48, P < .00001), and the difference was statistically significant.
Figure 2.
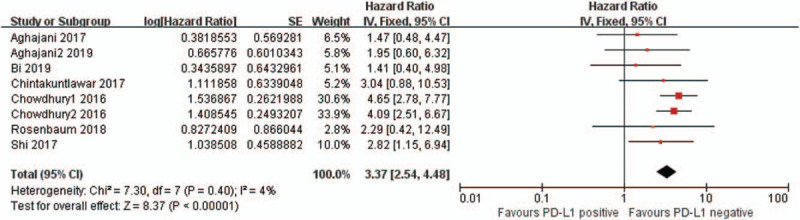
Forest plot describing the association between PD-L1 and disease-free survival (DFS) of thyroid cancer. PD-L1 = programmed cell death ligand 1.
3.5. PD-L1 expression can be applied as a biomarker to predict OS
Three articles[22,23,26] (3 studies) provided HRs and 95% CIs of OS in patients with thyroid cancer. As shown in Fig. 3, there was no evident heterogeneity among these 3 articles (I2 = 0%, P = .61), and the fixed-effects model was implemented. The meta-analysis revealed that positive PD-L1 expression was related to reduced OS in patients with thyroid cancer (HR = 2.52, 95% CI: 1.20–5.32, P = .01), and the difference was statistically significant. However, the study on OS only includes 3 articles, which may affect the final result; therefore, a higher number of studies with more patients is required for clarifying the role of PD-L1 in OS.
Figure 3.
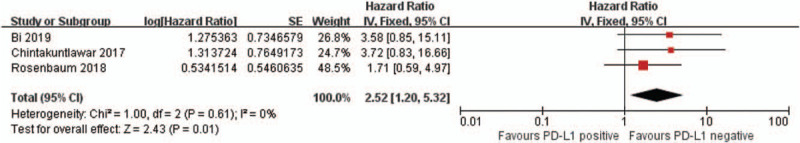
Forest plot describing the association between PD-L1 and overall survival (OS) of thyroid cancer. PD-L1 = programmed cell death ligand 1.
3.6. Relationship between PD-L1 and clinical pathology of thyroid cancer
Thirteen articles[17–29] (17 studies) provided the effect of positive and negative expression of PD-L1 on the clinicopathological features of patients with thyroid cancer. The results are presented in Table 2. The results revealed that PD-L1 overexpression was associated with tumor size ≥2 cm, tumor recurrence, extrathyroidal extension, concurrent thyroiditis, absence of psammoma body, and unifocal tumor (P < .05); however, PD-L1 expression was not related to age, sex, TNM stage, capsular invasion, lymphovascular invasion, calcification, laterality, and BRAFV600E mutation (P > .05).
Table 2.
The results of meta-analysis in thyroid cancer.
| Heterogeneity | Effect model | Publication bias | ||||||||
| Parameters | No. of studies | Groups | HR/OR, 95% CI | Significance (P value) | I2 (%) | P | Funnel plot | Begg | Egger | |
| DFS[17,18,22–24,26,27] | 8 | – | 3.37 (2.54, 4.48) | <.00001 | 4 | .40 | Fixed | Symmetry | 0.266 | 0.007 |
| OS[22,23,26] | 3 | – | 2.52 (1.20, 5.32) | .01 | 0 | .61 | Fixed | Symmetry | 0.296 | 0.045 |
| Age[17,19–21,27,29] | 6 | ≥45 or <45 | 0.85 (0.63, 1.13) | .27 | 0 | .77 | Fixed | Symmetry | 0.024 | 0.031 |
| Gender[17,18,20–23,25–29] | 15 | Male or female | 1.13 (0.78, 1.65) | .52 | 56 | .004 | Random | Symmetry | 0.882 | 0.888 |
| Tumor size[17–21,25,27,28] | 11 | ≥2 cm or <2 cm | 1.32 (1.03, 1.69) | .03 | 0 | .54 | Fixed | Symmetry | 1.000 | 0.238 |
| Recurrence[17,19,27] | 3 | Positive or negative | 1.92 (1.13, 3.26) | .02 | 0 | .59 | Fixed | Symmetry | 0.296 | 0.655 |
| TNM stage[18–24,28,29] | 12 | III–IV or I–II | 1.57 (0.78, 3.14) | .21 | 85 | <.00001 | Random | Symmetry | 0.222 | 0.380 |
| Extrathyroidal extension[17–21,25–28] | 12 | Positive or negative | 1.61 (1.24, 2.10) | .0003 | 10 | .35 | Fixed | Symmetry | 0.337 | 0.580 |
| Capsular invasion[18,22,24] | 7 | Positive or negative | 0.83 (0.33, 2.08) | .69 | 83 | <.00001 | Random | Symmetry | 0.453 | 0.545 |
| Lymphovascular invasion[17,18,28] | 6 | positive or negative | 1.10 (0.73, 1.66) | .65 | 30 | .21 | Fixed | Symmetry | 0.573 | 0.353 |
| Multifocality[17–22,24–29] | 15 | Multifocal or unifocal | 0.75 (0.61, 0.94) | .01 | 0 | .66 | Fixed | Symmetry | 0.729 | 0.482 |
| Thyroiditis[17–19,21,24–29] | 14 | Present or absent | 1.41 (1.10, 1.80) | .007 | 27 | .17 | Fixed | Symmetry | 0.511 | 0.629 |
| Psamomma body[20,21,29] | 3 | Present or absent | 0.34 (0.21, 0.55) | <.0001 | 0 | .97 | Fixed | Symmetry | 1.000 | 0.646 |
| Stromal calcification[20,21,29] | 3 | Present or absent | 0.87 (0.56, 1.36) | .54 | 17 | .30 | Fixed | Symmetry | 1.000 | 0.591 |
| BRAFV600E mutation[19–21] | 3 | Mutated or wild type | 1.83 (0.82, 4.09) | .14 | 59 | .09 | Random | Symmetry | 1.000 | 0.922 |
| Bilateral distribution[22,28] | 2 | Unilateral or bilateral distribution | 0.83 (0.43, 1.60) | .58 | 0 | .44 | Fixed | Symmetry | 1.000 | — |
TNM = tumor-nodes-metastases.
3.6.1. Age
Six articles[17,19–21,27,29] (6 studies) provided the effect of positive or negative expression of PD-L1 on the age of patients with thyroid cancer.. There was no evident heterogeneity among these 6 studies (I2 = 0%, P = .77), and the fixed-effects model was applied. According to the meta-analysis, there was no significant relationship between the age of patients and thyroid cancer (HR = 0.85, 95% CI: 0.63–1.13, P = .27).
3.6.2. Gender
Twelve articles[17,18,20–23,25–29] (15 studies) reported sex differences among patients with thyroid cancer with positive or negative expression of PD-L1. There was some heterogeneity among these 15 studies (I2 = 56%, P = .004), and the random-effects model was applied. The meta-analysis showed that the expression of PD-L1 was not associated with sex of patients with thyroid cancer (HR = 1.13, 95% CI: 0.78–1.65, P = .52).
3.6.3. Tumor size
Eight articles[17–21,25,27,28] (11 studies) provided the effect of positive or negative expression of PD-L1 on tumor size of patients with thyroid cancer. There was no evident heterogeneity among these 11 studies (I2 = 0%, P = .54), and the fixed-effects model was used. According to the meta-analysis, PD-L1 overexpression was associated with tumor size ≥2 cm (HR = 1.32, 95% CI: 1.03–1.69, P = .03), and the difference was statistically significant.
3.6.4. Recurrence
Three studies[17,19,27] (3 studies) provided the effect of positive or negative expression of PD-L1 on tumor recurrence in patients with thyroid cancer. There was no evident heterogeneity among these 3 studies (I2 = 0%, P = .59), and the fixed-effects model was implemented. The meta-analysis showed that PD-L1 overexpression had a relationship with tumor recurrence of thyroid cancer (HR = 1.92, 95% CI: 1.13–3.26, P = .02), and the difference was statistically significant.
3.6.5. TNM stage
Nine articles[18–24,28,29] (12 studies) provided the effect of positive or negative expression of PD-L1 on the TNM stage of patients with thyroid cancer. There was some heterogeneity among these 12 studies (I2 = 85%, P < .00001), and the random-effects model was applied. According to the analysis, PD-L1 was not significantly associated with TNM shield installation (HR = 1.57, 95% CI: 0.78–3.14, P = .21).
3.6.6. Extrathyroidal extension
Nine articles[17–21,25–28] (12 studies) provided the effect of positive or negative expression of PD-L1 on extrathyroidal extension in patients with thyroid cancer. There was no evident heterogeneity among these 12 studies (I2 = 10%, P = .35), and the fixed-effects model was implemented. The meta-analysis revealed that PD-L1 overexpression was related to the extrathyroidal extension of thyroid cancer (HR = 1.61, 95% CI: 1.24–2.10, P = .0003), and the difference was statistically significant.
3.6.7. Capsular invasion
Three articles[18,22,24] (7 studies) provided the effect of positive or negative expression of PD-L1 on the capsular invasion in patients with thyroid cancer. There was some heterogeneity among these 7 studies (I2 = 83%, P < .00001), and the random-effects model was applied. The meta-analysis revealed that PD-L1 expression was not associated with capsular invasion of thyroid cancer (HR = 0.83, 95% CI: 0.33–2.08, P = .69).
3.6.8. Lymphovascular invasion
Three articles[17,18,28] (6 studies) provided the effect of positive or negative expression of PD-L1 on lymphovascular invasion in patients with thyroid cancer. There was no evident heterogeneity among these 6 studies (I2 = 30%, P = .21), and the fixed-effects model was implemented. The meta-analysis showed that PD-L1 expression was not related to the lymphovascular invasion of thyroid cancer (HR = 1.10, 95% CI: 0.73–1.66, P = .65).
3.6.9. Multifocality
Eleven articles[17–22,24–28] (15 studies) provided the effect of positive or negative expression of PD-L1 on tumor multifocality of patients with thyroid cancer. There was no evident heterogeneity among these 15 studies (I2 = 0%, P = .66), and the fixed-effects model was implemented. The meta-analysis revealed that PD-L1 overexpression was related to the unifocal tumors of thyroid cancer (HR = 0.75, 95% CI: 0.061–.94, P = .01), and the difference was statistically significant.
3.6.10. Thyroiditis
Ten articles[17–19,21,24–29] (14 studies) provided the effect of positive or negative expression of PD-L1 in thyroid cancer complicated with thyroiditis. There was no heterogeneity among these 14 studies (I2 = 27%, P = .17), and the fixed-effects model was implemented. Meta-analysis indicated that high PD-L1 expression was related to thyroid cancer. (HR = 1.41, 95% CI: 1.10–1.80, P = .007), and the difference was statistically significant.
3.6.11. Psammoma body
Three articles[20,21,29] (3 studies) provided the effect of positive or negative expression of PD-L1 on the thyroid cancer-associated psammoma body. There was no evident heterogeneity among these 3 studies (I2 = 0%, P = .97), and the fixed-effects model was implemented. The meta-analysis revealed that PD-L1 overexpression was related to the absence of the psammoma body in patients with thyroid cancer (HR = 0.34, 95% CI: 0.21–.55, P < .0001), and the difference was statistically significant.
3.6.12. Stromal calcification
Three articles[20,21,29] (3 studies) provided the effect of positive or negative expression of PD-L1 on thyroid cancer-associated stromal calcification. There was no evident heterogeneity among these 3 studies (I2 = 17%, P = .30), and the fixed-effects model was applied. The meta-analysis showed that PD-L1 expression was not related to stromal calcification of thyroid cancer (HR = 0.87, 95% CI: 0.56–1.36, P = .54).
3.6.13. BRAFV600E mutation
Three studies[19–21] (3 studies) provided the effect of positive or negative expression of PD-L1 on BRAFV600E mutation in thyroid cancer. There was some heterogeneity among these 3 studies (I2 = 59%, P = .09), and the random-effects model was applied. The meta-analysis showed that PD-L1 expression was not related to BRAFV600E mutation in thyroid cancer (HR = 1.83, 95% CI: 0.82–4.09, P = .14).
3.6.14. Bilateral distribution
Two articles[22,28] (2 studies) provided the effect of positive or negative expression of PD-L1 on the unilateral and bilateral distribution of MTC. There was no evident heterogeneity among these 2 studies (I2 = 0%, P = .44), and the fixed-effects model was implemented. The meta-analysis revealed that PD-L1 expression was not related to the unilateral and bilateral distribution of MTC (HR = 0.83, 95% CI: 0.43–1.60, P = .58).
3.7. Subgroup analysis
Positive PD-L1 expression was related to poor DFS of DTC (HR = 3.61, 95% CI: 2.66–4.90, P = .00001) and DPTC + ATC (HR = 2.75, 95% CI: 1.01–7.50, P = .05). No distinct correlation was observed between PD-L1 expression and DFS of MTC (HR = 1.41, 95% CI: 0.40–4.98, P = .59). Overexpression of PD-L1 in DTC was related to tumor recurrence, concurrent thyroiditis, extrathyroidal extension, unifocal DTC, late stage of DTC, and BRAFV600E mutation in DTC; however, this correlation was not observed with age, sex, tumor size, capsular invasion, lymphovascular invasion, stromal calcification, and psammoma body of DTC. Subgroup analysis for bilateral distribution was not performed because this study only included MTC.
Subgroup analysis based on tumor origin confirmed that PD-L1-positive expression was related to poor DFS of thyroid cancer in the United States (HR: 2.75, 95% CI: 1.01–7.50, P = .05), China (HR = 2.23, 95% CI: 1.07–4.65, P = .03), and Canada (HR = 4.35, 95% CI: 3.05–6.19, P = .00001). There was no relationship between PD-L1 expression and DFS of thyroid cancer in Australia (HR = 1.68, 95% CI: 0.75–3.77, P = .21).
3.8. Heterogeneity analysis
The results of the Q-test demonstrated that there was statistical heterogeneity in sex, TNM stage, capsular invasion, and BRAFV600E mutation (I2 > 50%, P < .10); a random-effects model was employed in these studies, and no significant heterogeneity in statistics was found. Galbraith heterogeneity source analysis was performed for the 4 studies with heterogeneity. As shown in Fig. 4A, the source of heterogeneity in sex might be the studies conducted by Aghajani1 2019, Aghajani3 2019, and Bai 2017; after the deletion of these 3 studies, the heterogeneity decreased significantly (I2 = 35%, P = .11), confirming that these 3 studies were the sources of heterogeneity. Then, the fixed-effects model was employed for sex, and the results presented no noticeable change (OR = 1.11, 95% CI: 0.86–1.44, P = .42), indicating that the original findings were reliable. As shown in Fig. 4B, the source of heterogeneity in TNM stage may be the studies conducted by Chowdhury1 2016, Chowdhury2 2016, and Zhou 2019; after the deletion of these 3 studies, the heterogeneity decreased significantly (I2 = 0%, P = .56), confirming that these 3 studies were the sources of heterogeneity. Then, the fixed-effects model was applied, and the results showed no significant change (OR = 1.17, 95% CI: 0.86–1.60, P = .31), indicating that the original findings were reliable. For capsular invasion and BRAFV600E mutation, the Galbraith diagram could not identify the source of heterogeneity, which might be because of the small number of included articles. In addition, the difference between the sources of PD-L1 (e.g., membrane or cytoplasm), positive cutoff value, and types of PD-L1 clones in different studies may have affected the outcomes.
Figure 4.
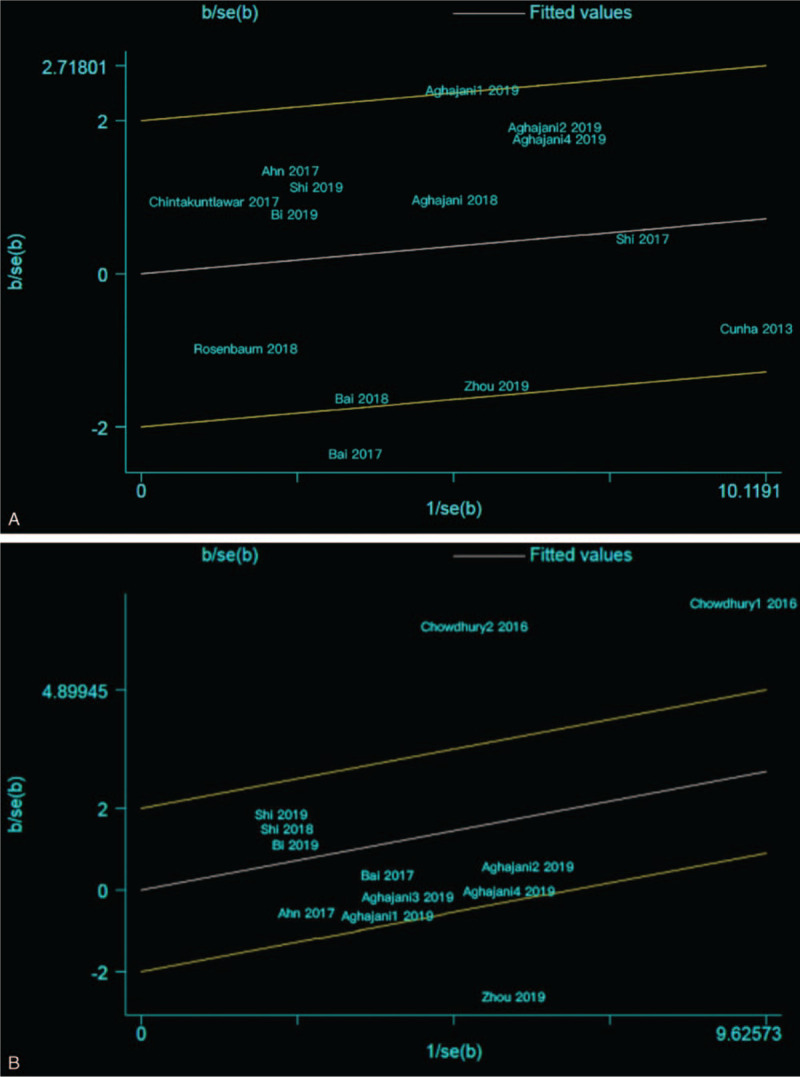
(A) Galbraith heterogeneity source analysis for sex: the source of heterogeneity in sex are the studies of Aghajani1 2019, Aghajani3 2019, and Bai 2017; B, Galbraith heterogeneity source analysis for TNM stage, the source of heterogeneity in TNM stage may be the studies of Chowdhury1 2016, Chowdhury2 2016, and Zhou 2019. TNM = tumor-nodes-metastases.
3.9. Sensitivity analysis
Sensitivity analysis was implemented by eliminating studies one by one using Stata15.1 to determine whether a single study could alter the integral results of DFS and OS. The results showed that no single study had a profound impact on the overall results, thus indicating that the analysis was relatively credible and stable (Fig. 5A and B).
Figure 5.
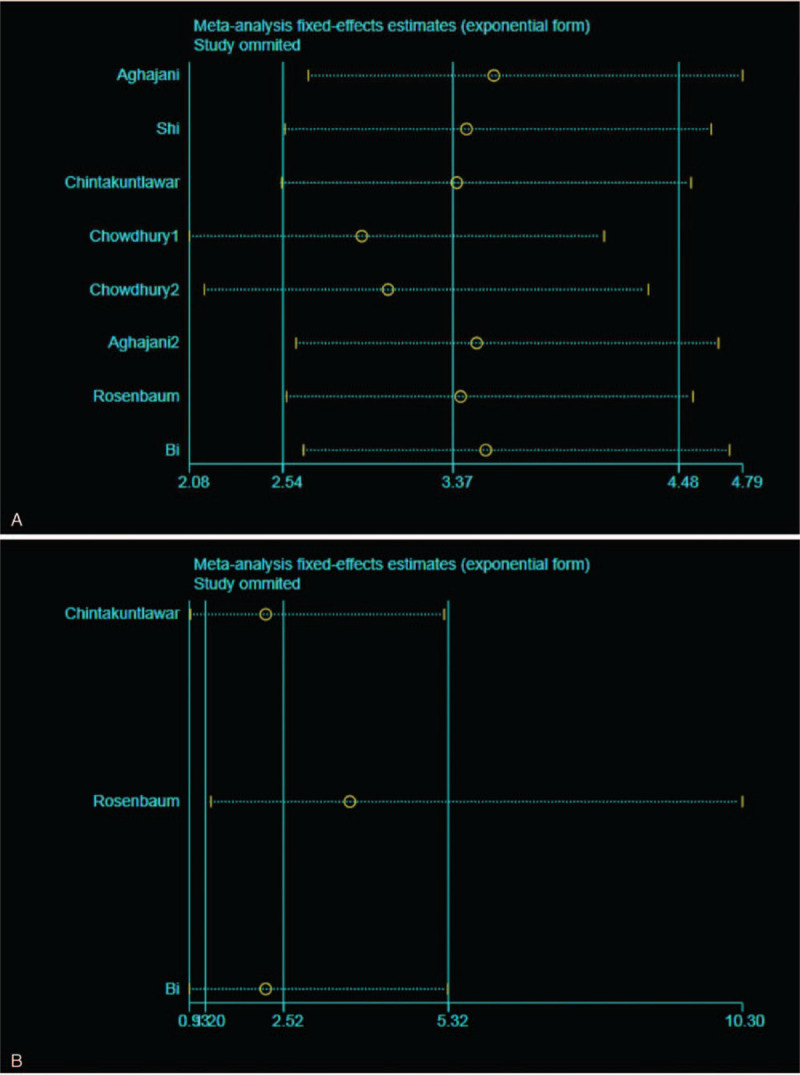
(A) Sensitivity analysis of DFS in thyroid cancer, individual study had little influence on the final disease-free survival (DFS); B, Sensitivity analysis of overall survival (OS) in thyroid cancer, individual study had little influence on the final OS.
3.10. Publication bias
We inspected publication bias using Egger and Begg tests, and the results are shown in Table 2. Egger and Begg tests indicated that there might have been publication bias in DFS, OS, and age. Therefore, these results still require further research to demonstrate their correctness.
4. Discussion
The PD-1/PD-L1 pathway exerts a negative effect on tumor immunoreaction, which can help tumor cells escape immune system surveillance by promoting T cell apoptosis and inhibiting lymphocyte and T cell proliferation, leading to poor prognosis of tumors. PD-L1 is abnormally expressed in many malignant tumors and can be used as a prognostic marker for malignancy. However, the association between PD-L1 expression and thyroid cancer prognosis remains controversial. The research by Shi et al[28] demonstrated that thyroid cancer with late TNM stage, tumor size ≥2 cm, and positive lymph node metastasis showed high expression of PD-L1, and the expression of PD-L1 was not related to patients’ sex, extrathyroidal extension, concurrent thyroiditis, multifocality, and lymphovascular invasion. The 5-year recurrence-free survival of negative and positive expression of PD-L1 were 85.4% and 57.9%, respectively (P = .001). In contrast, another study concluded that PD-L1 expression was significantly associated with extrathyroidal extension, concurrent thyroiditis, and lymphovascular invasion but not with multifocality, TNM stage, and lymph node metastasis, and there was no association between the increase in PD-L1 expression and the decrease in DFS (P = .266).[17]
According to the findings of this meta-analysis, PD-L1 appeared to be a biomarker to evaluate survival outcomes of patients with thyroid cancer, suggesting that patients with higher expression of PD-L1 had poor DFS and OS (P < .05), and there was no evident heterogeneity among studies of DFS and OS. The results on DFS are consistent with those reported in 2 previous meta-analyses,[15,16] Subgroup analysis showed that a positive PD-L1 expression was associated with a poor DFS for DTC and DPTC + ATC, and there was no heterogeneity; however, this correlation was not found between PD-L1 expression and DFS of MTC. In general, the positive expression of PD-L1 was closely related to follicular epithelial thyroid cancer. In other words, the positive expression of PD-L1 seems to indicate that the type of thyroid cancer originates from thyroid follicular epithelial cells. Lin et al[30] reported that acting at a cell surface receptor on plasma membrane integrin αvβ3, thyroxine produced by thyroid follicular epithelial cells stimulates intracellular accumulation of PD-L1 in cancer cells and induces downstream signaling pathways that promote tumor cell proliferation, such as mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase and phosphatidylinositol-3-kinase/protein kinase B pathways, which can promote PD-L1 overexpression. Our results on OS differ from those of Girolami study[16]; unfortunately, our study on OS only included 3 articles, which may have affected the final result. Therefore, a large number of studies with more patients are needed to clarify the association between PD-L1 expression and OS.
This meta-analysis also showed that high PD-L1 expression was associated with tumor size ≥2 cm, tumor recurrence, extrathyroidal extension, concurrent thyroiditis, absence of psammoma body, and unifocal thyroid cancer (P < .05), and there was no apparent correlation between PD-L1 expression and age, sex, TNM stage, capsular invasion, lymphovascular invasion, stromal calcification, bilateral distribution, or BRAFV600E mutation (P > .05). According to the pooled data, overexpression of PD-L1 was more significantly associated with advanced tumors, lymphovascular invasion, and BRAFV600E mutation, although not statistically significant; these findings might provide valuable insights for future clinical work. Subgroup analysis was performed according to different types of thyroid cancer and showed that positive PD-L1 expression in DTC was related to tumor recurrence, concurrent thyroiditis, extrathyroidal extension, unifocal DTC, late stage of DTC, and BRAFV600E mutation in DTC; however, no correlation was observed with age, sex, tumor size, capsular invasion, lymphovascular invasion, stromal calcification, and psammoma body of DTC. In many experiments, the relationship between PD-L1 expression and BRAFV600E remains controversial. BRAFV600E tumors more often express high levels of PD-L1 compared with BRAF wild-type tumors (53% vs 12.5%), as reported by Angell et al.[31] The research by Brauner et al[32] demonstrated that compared with BRAFV600E wild type, BRAFV600E-mutated patients had a higher level of PD-L1 mRNA (P = .015). However, few studies[21,33] reported the opposite conclusion that PD-L1 expression was not related to BRAF mutations. In our meta-analysis, overexpression of PD-L1 in DTC was more significant in patients with BRAFV600E mutation, which should be further verified to determine whether there is an association with other types of thyroid cancer. Relative to the meta-analysis in 2020,[16] our meta-analysis assessed the relationship between PD-L1 expression and bilateral tumor distribution, stromal calcification, psammoma body, and capsular invasion. The absence of psammoma bodies was also associated with the overexpression of PD-L1 (HR = 0.34, 95% CI: 0.21–0.55, P < .0001), and no evident heterogeneity among studies on psammoma bodies (I2 = 0%, P = .97) was observed. Although there was no correlation between these bilateral tumor distributions, stromal calcification, and capsular invasion and the expression of PD-L1 (P > .05), this meta-analysis may provide some useful insights for subsequent studies, and we plan to further discuss these studies in future articles.
Blocking the PD-1/PD-L1 signaling pathway is important for the treatment of malignant tumors and has shown outstanding efficacy in studies on melanoma, non-small cell lung carcinoma, head and neck squamous cell carcinoma, urothelial carcinoma, renal cell carcinoma, and breast carcinoma.[34–38] The prognosis of refractory aggressive thyroid cancer is poor, and standard treatment and tyrosine kinase inhibitors may not be valid options. Currently, there is an urgent need to improve the survival outcome of patients through immune biomarkers and to improve refractory thyroid cancer by obstructing the PD-1/PD-L1 pathway. Immunotherapy has not been approved by the Food and Drug Administration for refractory thyroid cancer, but few studies on ATC animal models have shown remarkable efficacy. Gunda et al[39] found that PD-1/PD-L1 inhibitor combined with BRAF inhibitor (PLX4720) could prominently reduce tumor volume, prolong survival, and improve immune ability in tumors of ATC mice. Brauner et al[32] reached a similar conclusion. In the tumor microenvironment with high expression of PD-L1, the MAPK activity of immune cells decreases, resulting in decreased cytokine secretion. With the use of BRAF inhibitor PLX4032, the MAPK activity of immune cells increased. The production of tumor necrosis factor-γ inhibited the expression of PD-L1 mRNA, and BRAF inhibitors also inhibited the expression of PD-L1. The combination of BRAF inhibitor and PD-L1 blocking treatment can increase the CD8+/Treg cell ratio, increase the production of cytokines, enhance the cytotoxicity and anti-tumor immune response of T cells, and ultimately destroy tumor cells. Another study conducted by Gunda et al[40] that combined lenvatinib and anti-PD-1 monoclonal antibody to treat ATC mice showed that the combination therapy could ameliorate lenvatinib's expansion of myeloid-derived suppressor cells and dramatically improve lenvatinib's anti-tumor effect. Aghajani et al[41] presented a case of a patient with refractory and advanced ATC who was treated with an anti-PD-1 monoclonal antibody. A remarkable increase in the population of T cells, B cells, and NK cells was observed after the treatment. Twenty-two terminal patients with ATC were included in the trial by Mehnert et al[42] to estimate the anti-tumor ability of pembrolizumab. This study verified that 2 of 22 patients showed a partial response and 9% showed an overall response, and median progression-free survival was 7 months; however, 82% had treatment-related adverse events. Currently, many clinical trials are underway to evaluate the efficacy and safety PD-1/PD-L1 inhibitors in thyroid cancer (including NCT02688608, NCT03181100, and NCT03246958).
However, this study has some limitations: the number of included studies was limited, the information provided was limited, and some studies only included 2 to 3 articles, which may have affected the research results; no subgroup analysis was performed on positive cutoff values of PD-L1 and different treatment regimens, which may have led to heterogeneity in the overall results; there was a publication bias regarding DFS, OS, and patient age; hence, future studies are needed to verify the results of this meta-analysis; the studies included in this meta-analysis were mainly from China, Australia, the United States, and Canada, and the conclusions may be biased due to regional or ethnic factors; and the difference in terms of the sources of PD-L1 (e.g., membrane or cytoplasm), reagents of IHC, the positive cutoff value of PD-L1, sample sizes, and the types of PD-L1 clones in various studies may have affected the outcomes. For the detection of PD-L1, next-generation sequencing technologies[43] and Raman-enhanced spectroscopy[44] probes are expected to be applied. Despite the above shortcomings, this meta-analysis revealed the relationship between PD-L1 overexpression and the survival and clinicopathological characteristics of patients with thyroid cancer, which may contribute to the stratification of thyroid cancer, thus improving the curative efficacy of PD-1/PD-L1 inhibitors.
5. Conclusion
In this study, we found that the positive effect of PD-L1 expression in patients with thyroid cancer was associated with low survival. High PD-L1 expression was associated with tumor size ≥2 cm, tumor recurrence, extrathyroidal extension, concurrent thyroiditis, absence of psammoma body, and unifocal thyroid cancer, which can help clinicians predict the recurrence of thyroid cancer and select a more appropriate treatment plan for patients with thyroid cancer. Analysis of the sub-groups revealed that the positive expression PD-L1 is associated with poor prognosis of DFS of DTC and DPTC + ATC, and the overexpression of PD-L1 in DTC was related to tumor recurrence, concurrent thyroiditis, extrathyroidal extension, unifocal DTC, late stage of DTC, and BRAFV600E mutation in DTC. These findings might provide useful insights for future clinical studies on DTC. Molecular targeted therapy drugs such as PD-1/PD-L1 inhibitors and BRAF inhibitors may provide new treatment regimens for patients with thyroid cancer exhibiting poor outcomes with conventional treatment methods and contribute to the clinical improvement of the individual treatment for patients. However, extensive homogeneous studies are needed to validate these findings.
Acknowledgments
The authors would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Software: Baoyu Wan, Pengyi Deng.
Supervision: Wenli Dai.
Writing – original draft: Baoyu Wan, Pengyi Deng, Zhizhi Dong.
Writing – review & editing: Pengyi Deng, Wenli Dai, Peng Wang, Chaojun Yang, Jinling Tian, Tao Hu, Kai Yan.
Footnotes
Abbreviations: ATC = anaplastic thyroid carcinoma, CI = confidence interval, DFS = disease-free survival, DTC = differentiated thyroid carcinoma, FTC = follicular thyroid carcinoma, HR = hazard ratio, IHC = immunohistochemistry, MAPK = mitogen-activated protein kinase, MTC = medullary thyroid carcinoma, NOS = Newcastle-Ottawa Quality Assessment Scale, OR = odds ratio, OS = overall survival, PD-1 = programmed cell death receptor 1, PD-L1 = programmed cell death ligand 1, PDTC = poorly differentiated thyroid carcinoma, PTC = papillary thyroid cancer, TNM = tumor-nodes-metastases.
How to cite this article: Wan B, Deng P, Dai W, Wang P, Dong Z, Yang C, Tian J, Hu T, Yan K. Association between programmed cell death ligand 1 expression and thyroid cancer: a meta-analysis. Medicine. 2021;100:14(e25315).
BW and PD contributed equally to this study.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013;2013:965210–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941–53. [DOI] [PubMed] [Google Scholar]
- [3].Gomez AML, Santana PC, Mourão AP. Dosimetry study in head and neck of anthropomorphic phantoms in computed tomography scans. SciMed J 2020;2:38–43. [Google Scholar]
- [4].Abdelaal AM, Attalla EM, Elshemey WM. Estimation of out-of-field dose variation using markus ionization chamber detector. SciMed J 2020;2:08–15. [Google Scholar]
- [5].Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet 2016;388:2783–95. [DOI] [PubMed] [Google Scholar]
- [6].Chmielik E, Rusinek D, Oczko-Wojciechowska M, et al. Heterogeneity of thyroid cancer. Pathobiology 2018;85:117–29. [DOI] [PubMed] [Google Scholar]
- [7].Sanders EJ, LiVolsi VA, Brierley J, et al. An evidence-based review of poorly differentiated thyroid cancer. World J Surg 2007;31:934–45. [DOI] [PubMed] [Google Scholar]
- [8].Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–9. [DOI] [PubMed] [Google Scholar]
- [9].Prasongsook N, Kumar A, Chintakuntlawar AV, et al. Survival in response to multimodal therapy in anaplastic thyroid cancer. J Clin Endocrinol Metab 2017;102:4506–14. [DOI] [PubMed] [Google Scholar]
- [10].Seliger B. Basis of PD1/PD-L1 therapies. J Clin Med 2019;8:2168–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Williams M, Lidke DS, Hartmann K, et al. PD-L1 expression in mastocytosis. Int J Mol Sci 2019;20:2362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 2019;18:10–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lenouvel D, Gonzalez-Moles MA, Ruiz-Avila I, et al. Prognostic and clinicopathological significance of PD-L1 overexpression in oral squamous cell carcinoma: a systematic review and comprehensive meta-analysis. Oral Oncol 2020;106:104722–32. [DOI] [PubMed] [Google Scholar]
- [14].Qi Y, Xia Y, Lin Z, et al. Tumor-infiltrating CD39(+)CD8(+) T cells determine poor prognosis and immune evasion in clear cell renal cell carcinoma patients. Cancer Immunol Immunother 2020;69:1565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aghajani M, Graham S, McCafferty C, et al. Clinicopathologic and prognostic significance of programmed cell death ligand 1 expression in patients with non-medullary thyroid cancer: a systematic review and meta-analysis. Thyroid 2018;28:349–61. [DOI] [PubMed] [Google Scholar]
- [16].Girolami I, Pantanowitz L, Mete O, et al. Programmed death-ligand 1 (PD-L1) is a potential biomarker of disease-free survival in papillary thyroid carcinoma: a systematic review and meta-analysis of PD-L1 immunoexpression in follicular epithelial derived thyroid carcinoma. Endocr Pathol 2020;31:291–300. [DOI] [PubMed] [Google Scholar]
- [17].Aghajani MJ, Yang T, McCafferty CE, et al. Predictive relevance of programmed cell death protein 1 and tumor-infiltrating lymphocyte expression in papillary thyroid cancer. Surgery 2018;163:130–6. [DOI] [PubMed] [Google Scholar]
- [18].Aghajani MJ, Roberts TL, Yang T, et al. Elevated levels of soluble PD-L1 are associated with reduced recurrence in papillary thyroid cancer. Endocr Connect 2019;8:1040–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ahn S, Kim TH, Kim SW, et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer 2017;24:97–106. [DOI] [PubMed] [Google Scholar]
- [20].Bai Y, Guo T, Huang X, et al. In papillary thyroid carcinoma, expression by immunohistochemistry of BRAF V600E, PD-L1, and PD-1 is closely related. Virchows Arch 2018;472:779–87. [DOI] [PubMed] [Google Scholar]
- [21].Bai Y, Niu D, Huang X, et al. PD-L1 and PD-1 expression are correlated with distinctive clinicopathological features in papillary thyroid carcinoma. Diagn Pathol 2017;12:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bi Y, Ren X, Bai X, et al. PD-1/PD-L1 expressions in medullary thyroid carcinoma: clinicopathologic and prognostic analysis of Chinese population. Eur J Surg Oncol 2019;45:353–8. [DOI] [PubMed] [Google Scholar]
- [23].Chintakuntlawar AV, Rumilla KM, Smith CY, et al. Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study. J Clin Endocrinol Metab 2017;102:1943–50. [DOI] [PubMed] [Google Scholar]
- [24].Chowdhury S, Veyhl J, Jessa F, et al. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 2016;7:32318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cunha LL, Marcello MA, Morari EC, et al. Differentiated thyroid carcinomas may elude the immune system by B7H1 upregulation. Endocr Relat Cancer 2013;20:103–10. [DOI] [PubMed] [Google Scholar]
- [26].Rosenbaum MW, Gigliotti BJ, Pai SI, et al. PD-L1 and IDO1 are expressed in poorly differentiated thyroid carcinoma. Endocr Pathol 2018;29:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shi R, Qu N, Luo T, et al. Programmed death-ligand 1 expression in papillary thyroid cancer and its correlation with clinicopathologic factors and recurrence. Thyroid 2017;27:537–45. [DOI] [PubMed] [Google Scholar]
- [28].Shi X, Yu P, Lei B, et al. Association between programmed death-ligand 1 expression and clinicopathological characteristics, structural recurrence, and biochemical recurrence/persistent disease in medullary thyroid carcinoma. Thyroid 2019;29:1269–78. [DOI] [PubMed] [Google Scholar]
- [29].Zhou L, Cha G, Chen L, et al. HIF1α/PD-L1 axis mediates hypoxia-induced cell apoptosis and tumor progression in follicular thyroid carcinoma. Onco Targets Ther 2019;12:6461–70. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [30].Lin HY, Chin YT, Shih YJ, et al. In tumor cells, thyroid hormone analogues non-immunologically regulate PD-L1 and PD-1 accumulation that is anti-apoptotic. Oncotarget 2018;9:34033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Angell TE, Lechner MG, Jang JK, et al. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 2014;24:1385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brauner E, Gunda V, Vanden Borre P, et al. Combining BRAF inhibitor and anti PD-L1 antibody dramatically improves tumor regression and anti tumor immunity in an immunocompetent murine model of anaplastic thyroid cancer. Oncotarget 2016;7:17194–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bastman JJ, Serracino HS, Zhu Y, et al. Tumor-infiltrating T cells and the PD-1 checkpoint pathway in advanced differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab 2016;101:2863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- [36].Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- [37].Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. [DOI] [PubMed] [Google Scholar]
- [38].Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. [DOI] [PubMed] [Google Scholar]
- [39].Gunda V, Gigliotti B, Ndishabandi D, et al. Combinations of BRAF inhibitor and anti-PD-1/PD-L1 antibody improve survival and tumour immunity in an immunocompetent model of orthotopic murine anaplastic thyroid cancer. Br J Cancer 2018;119:1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gunda V, Gigliotti B, Ashry T, et al. Anti-PD-1/PD-L1 therapy augments lenvatinib's efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int J Cancer 2019;144:2266–78. [DOI] [PubMed] [Google Scholar]
- [41].Aghajani MJ, Cooper A, McGuire H, et al. Pembrolizumab for anaplastic thyroid cancer: a case study. Cancer Immunol Immunother 2019;68:1921–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mehnert JM, Varga A, Brose MS, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer 2019;19:196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kosvyra A, Maramis C, Chouvarda I. Developing an integrated genomic profile for cancer patients with the use of NGS data. Emerg Sci J 2019;3:157–67. [Google Scholar]
- [44].Agsalda-Garcia M, Shieh T, Souza R, et al. Raman-enhanced spectroscopy (RESpect) probe for childhood non-Hodgkin lymphoma. SciMed J 2020;2:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


