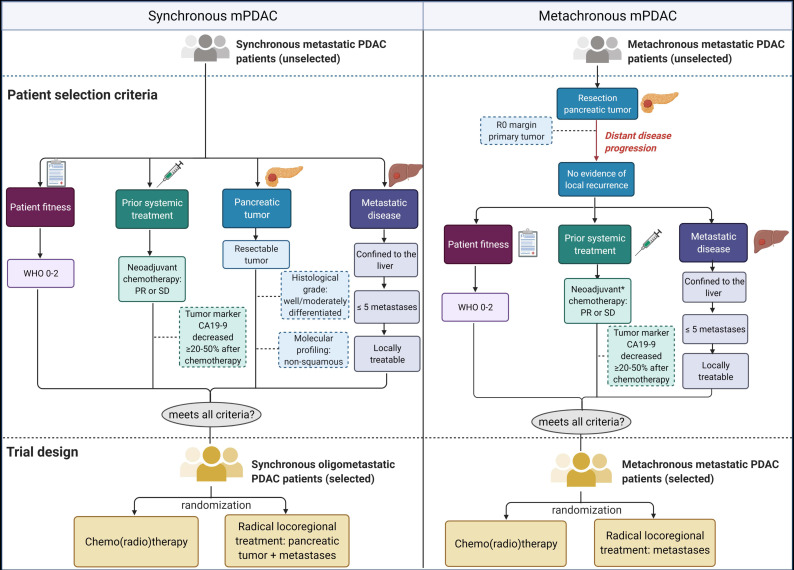
Figure 2.
Randomized controlled trial (RCT) design. Two potential setups of an RCT to determine the possible life-prolonging value of locoregional treatment in synchronous (left) or metachronous (right) metastatic PDAC (mPDAC). The RCTs adhere to four main selection pillars: patient fitness, prior systemic treatment, pancreatic tumor and metastatic disease. Selection criteria include WHO performance status 0–2, partial response (PR) or stable disease (SD) after neoadjuvant chemotherapy, having a resectable primary tumor (synchronous mPDAC), metastatic disease confined to the liver and ≤5 metastases that are locally treatable. For metachronous mPDAC, the pancreatic tumor has to be resected previously, without evidence of local recurrence. In addition to the main criteria, several supportive selection criteria are portrayed (dotted outline), which may or may not be used, including a decrease of ≥20–50% in tumor marker CA19-9 serum levels after chemotherapy, lower histological grade (well/moderately differentiated), non-squamous transcriptomic subtype and, in case of metachronous mPDAC, an R0 resection margin of the primary tumor. If mPDAC patients meet all these requirements, they can be randomized into either a radical locoregional treatment group or a control group receiving chemo(radio)therapy. * After primary resection but prior to metastatic treatment.