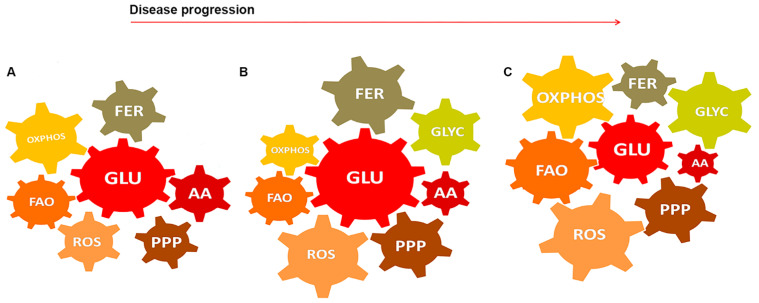
Figure 2.
Metabolic modules in the GBM-glial cell network during the disease progression. (A) Metabolic modules may be considered as gears regulating the engine performance, that is the bioenergetic machinery in biological tissues. Based on the literature review, we hypothesized the relationships between the metabolic modules in the GBM-glial cells network. In physiological conditions, glucose oxidation-derived products allow both the OXPHOS, FER, and PPP. The ROS production is counterbalanced by the AA pool, which is partially used for synthesizing anti-oxidant agents. Fatty acid oxidation also precedes ATP production and substrates for biosynthesis. (B) With the presence of GBM, metabolic and mitochondrial rewiring occurs. Both cancer and non-cancer cells are supposed to rely on glycolytic metabolism and PPP for ATP and biosynthesis need respectively. ROS production exceeds and impairs the mitochondrial OXPHOS. Astrocytes maintain the FER to fulfill the neurons’ metabolic needs. Glycogen and non-essential AA are recruited as an alternative source of glucose. (C) In the condition of glucose starvation, glycogen deposits and AA keep on fuel the energetic and biosynthetic metabolism [189]. FAO acquires a major role to sustain the OXPHOS, but the overproduction of ROS may trigger new adaptations and maladaptive plasticity. Glucose oxidation (glycolysis, GLU); anaerobic fermentation (FER), amino acids (AA), oxidative phosphorylation (OXPHOS); reactive oxygen species (ROS); pentose phosphate pathway (PPP); fatty acid oxidation (FAO); glycogenolysis (GLYC).