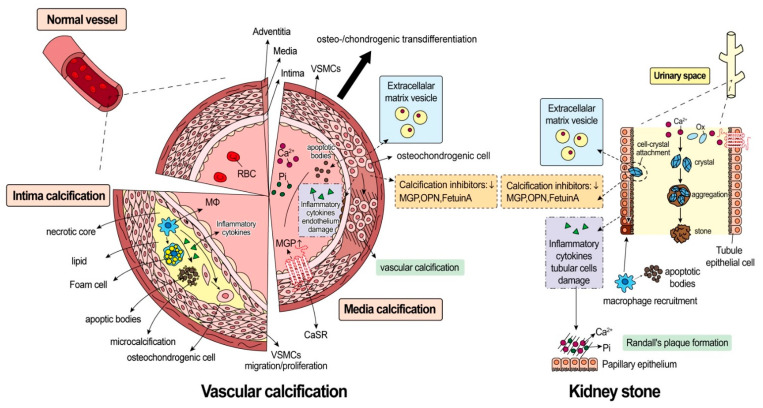
Figure 2.
Crosstalk between vascular calcification and nephrolithiasis. The process of vascular calcification is demonstrated on the right while that of kidney stone formation is depicted on the left. The molecular characteristics shared by these two distinct diseases are highlighted by the same-colored block. Vascular calcification can be categorized into intimal calcification and media calcification. 1. Intimal calcification is frequently associated with atherosclerosis. Macrophage (Mφ) digests lipoproteins and converts them to cholesterol-rich foam cells. Initially, foam cells can be phagocyted by adjacent vascular smooth muscle cells (VSMCs) and release apoptotic bodies. In addition to an increase in the number of apoptotic bodies, there is an accumulation of apoptotic body debris, which results in the formation of a necrotic core. Vesicles in the necrotic core release microcalcification of calcium phosphate as nucleation nidus. 2. Medial calcification is largely related to osteo-/chondrogenic transdifferentiation, which indicates the change in phenotype of VSMCs into osteo-/chondroblasts like cells. Transdifferentiation can be induced by high phosphate (Pi) or calcium (Ca2+) levels. The osteo-/chondroblast-like cells actively promote media calcification by reduced activities of calcification inhibitors (e.g., matrix Gla protein (MGP), osteopontin (OPN), and fetuin A), apoptotic body release, calcifying extracellular matrix vesicle release, and inflammatory cytokine release. The activation of CaSR on VSMCs can stimulate MGP release, which can ameliorate vascular calcification. The mechanism of kidney stone formation remains largely unknown. However, the supersaturated urinary stone promoters, e.g., Ca2+ and oxalate (Ox), can gradually form crystals, and aggregated crystals could interact with tubule epithelial cells and cause epithelial damage. The cell-crystal interaction could cause calcifying extracellular matrix vesicles release and inflammatory cytokines release, with the assistance of reduced activities of calcification inhibitors (e.g., MGP, OPN, and fetuin A). Mφ recruitment and polarization are also found to occur in the crystal-attached areas. Finally, Ca2+ and Pi combine to form hydroxyapatite, which is deposited on the renal papilla and referred to as Randall’s plaque.