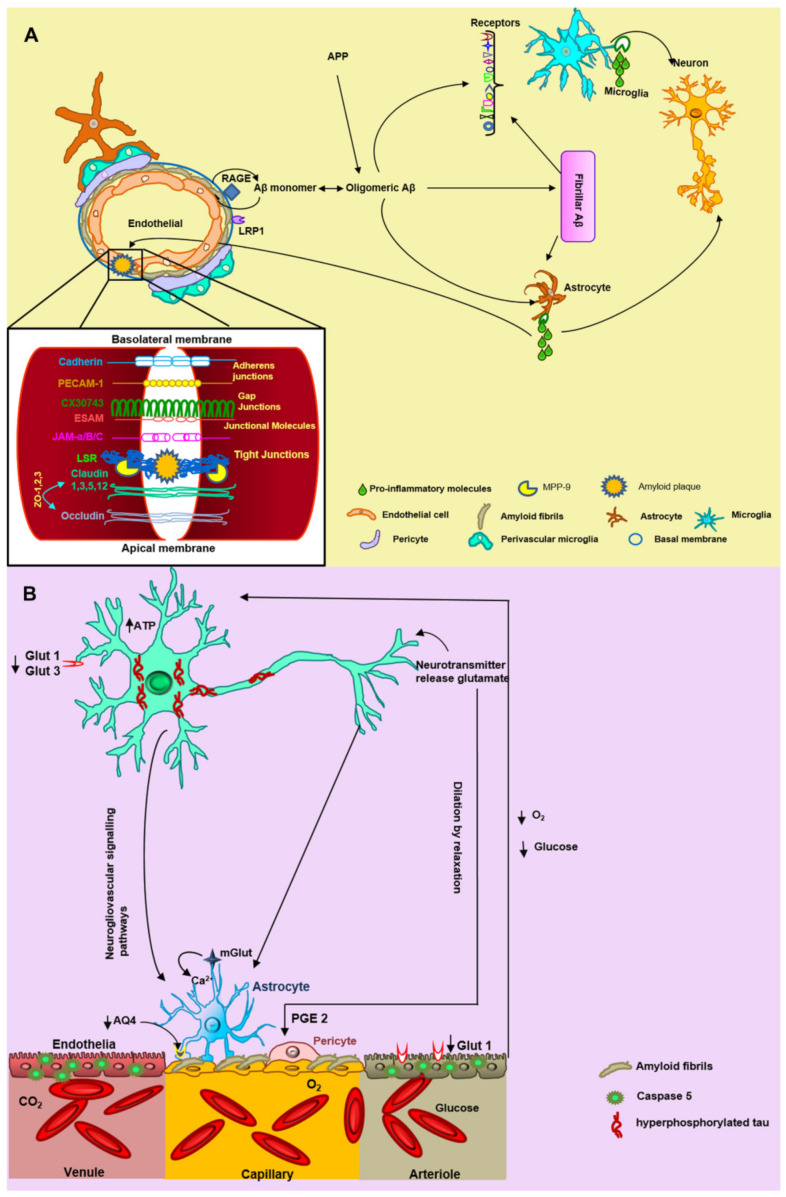
Figure 7.
The hypothesis of neurovascular unit (NVU) alteration triggered by Aβ fibrillary deposits. (A) Aβ monomers are incorporated into oligomers and fibrils in the brain, triggering NVU degeneration. Some endothelial receptors such as the receptor for advanced glycation end-products (RAGE) and low-density lipoprotein receptor-related protein 1 (LRP1) are involved in the vascular Aβ accumulation and thus with blood–brain barrier (BBB) damage. The microglial receptors TLR2, MARCO, SCARA ½, CR3 (β2 integrin), CR4, SCARB2, CD36, TLR4-TLR6, CD47, α6β1 integrin, SR-A1, and TREM 2 interact with fibrillar Aβ. Subsequently, microglia and astrocytes secrete pro-inflammatory molecules that promote neuronal and vascular damage. Fibrillar Aβ aggregates or NP could trigger the breaking of tight junctions specifically by metalloproteinase 9 (MMP9). (B) There is an accumulation of fibrillar Aβ in endothelial cells causing oligemia (decrease in oxygen and glucose levels). In the neuron, there are two important glucose receptors GLUT 1 and GLUT 3, which are decreased during Alzheimer’s disease, leading to phosphorylation of tau, excitotoxicity, and cell death. Various studies have found a decrease in aquaporin 4 (AQ4) in astrocytes, generating edema of the astrocytic feet. The pericytes undergo dilation by relaxation due to the presence of PGE 2. The neurotransmitter glutamate is released from the neuron to the astrocyte, where it produces an increase in intracellular calcium. We rely on the genome.jp/keg and expasy.org platforms and the results obtained in this study for the proposed hypothesis.