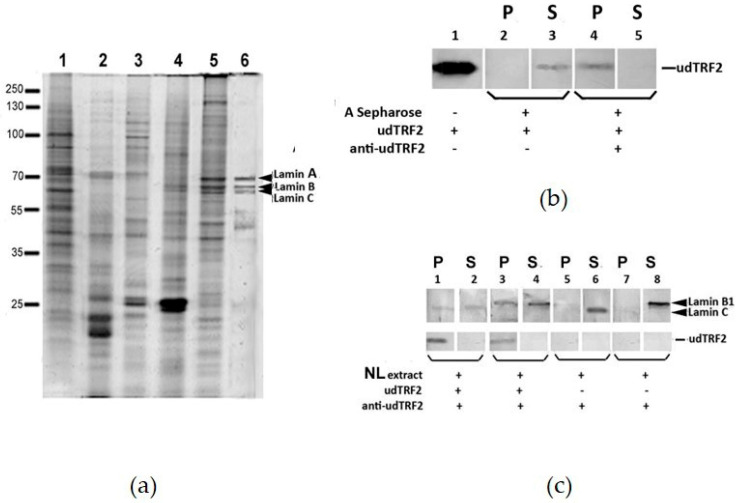
Figure 3.
(a) Nuclear lamina (NL) purification and extraction (12% SDS-PAGE, CBB). Lane 1, soluble fraction after Triton; lane 2, soluble fraction after nuclease; lane 3, soluble fraction after LS buffer; lane 4, soluble fraction after HS buffer; lane 5, nuclear matrix (NM) precipitate; lane 6, NL extract with lamins A, B, C marked with arrows. Mr of marker indicated on the left. (b) udTRF2 AB evaluation. Lane 1, recombinant udTRF2 protein loaded; lane 2, control sample without anti-udTRF2 AB, Sepharose eluate; binding absent; lane 3, sample without anti-udTRF2 antibodies, supernatant, binding absent; lane 4, sample with anti-udTRF2 antibodies, Sepharose eluate, binding present; lane 5, sample with anti-udTRF2 antibodies, supernatant, binding present. P and S, pellet and supernatant from protein A Sepharose column, respectively. The input for each experiment is shown underneath and underlined with brackets. Anti-TRF2 AB (ab4182, Abcam) was used for Western blotting. (c) CoIP (co-immunoprecipitation) results analyzed with anti-lamin C and B1 ABs in the upper row and with TRF2 AB in the lower row. P and S, pellet and supernatant from protein A Sepharose column, respectively. The input for each experiment is shown underneath the brackets. Lamin positions are marked by arrows; udTRF2 position is marked by a dash.