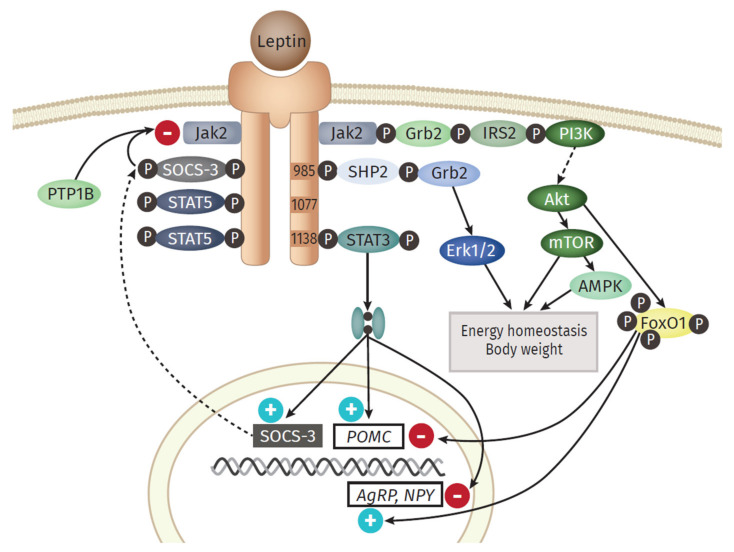
Figure 2.
Overview of the leptin signaling pathway. The long isoform of the leptin receptor (ObRb) possesses the whole intracellular domain, with the conserved tyrosine residues (Y985, Y1077, Y118). The central function of leptin in the hypothalamus is regulated via the activation of anorexigenic neurons and orexigenic Neuropeptide Y (NPY)/Agouti-related Protein (AgRP) neurons. The most prominent downstream signaling component of the leptin receptor is the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. After binding of leptin, subsequent dimerization of the receptor and the following activation of JAK2 occurs, followed by the recruitment of the Src homology 2 (SH2) domain of STAT3 to the conserved phosphorylated tyrosine Y705 residue. Hereinafter, STAT3 itself is phosphorylated by the JAKs at position Y705, dimerizes, and translocates into the nucleus. At this point, it serves as a regulator of the expression of different STAT3 responsive genes. One prominent downstream gene is the suppressor of cytokine signaling 3 (SOCS3), which itself acts as a potent negative regulator of the leptin signaling, by binding of Y985 domain and following inhibition of JAK2. Protein tyrosine phosphatase 1 B (PTP1B), produced in the endoplasmic reticulum (ER), is further able to inhibit leptin signaling by dephosphorylating JAK2. Besides STAT3, STAT5 is also known to be activated and phosphorylated in vivo. Another downstream signaling branch of leptin is the Mitogen-activated protein kinase (MAPK) pathway. Here, the SH2 domain of the phosphatase SH2- containing protein tyrosine phosphatase 2 (SHP2) binds to pY985, becomes phosphorylated by JAKs, which finally activates MAPK extracellular signal-related kinase (ERK1/2) via recruitment of growth factor receptor-bound protein 2 (Grb2). The phosphatidylinositol 3 kinase (PI3K) signaling pathway is activated by leptin. IRS 2 (insulin receptor substrate 2) binds to ObRb through the SH2B1 domain, able to interact and upregulate JAK2. Those IRS proteins are then capable of binding and activating PI3K yielding the subsequent accumulation of phosphatidylinositol 3,4,5-triphosphate (PIP3) and activation of 3-phosphoinositide- dependent protein kinase (PDK1) and Akt. The latter inhibits forkhead box O1 (Foxo1), the mediator of the previously mentioned function of leptin in the hypothalamus. Another component downstream of Akt is the Ser/Thr kinase mTOR (mammalian target of rapamycin), another sensor of the availability of nutrients and stimulator of cell growth, protein biosynthesis, and proliferation. The AMPK (adenosine monophosphate-activated protein kinase) pathway is stimulated by leptin as well, but the outcome differs between the tissues. While activated in hepatocytes and muscle, AMPK is inhibited in the hypothalamus, resulting in inhibition of food intake.