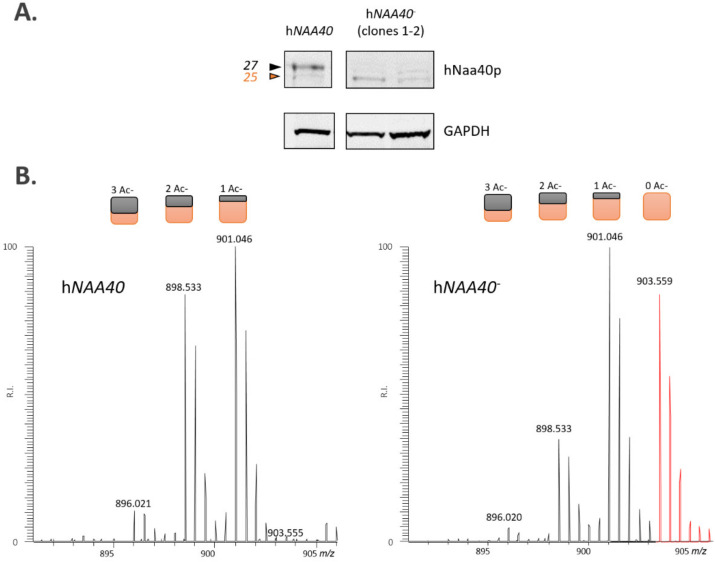
Figure 5.
Residual hNaa40S proteoform expression in hNAA40L- knockout HAP1 cells is deficient in maintaining Nt-acetylation of the hNaa40p substrate histone H4. (A) Western blotting analyses of corresponding HAP1 WT and hNAA40L- knockout HAP1 cell lysates (originating from two independent hNAA40 knockout clones) immunoblotted with antibodies specific for hNaa40p and GAPDH are shown. (B) Besides the identification of various N-acetylated forms of the mature N-terminus of human histone H4 (2SGRGKGGKGLGKGGAKR18), the fully N-free form (red MS trace) was only demonstrated in the hNAA40- setup as inferred from the doubly charged variant observed at a higher m/z (903.559). The 5 Da spacing between the distinct isotopic envelopes, corresponds with the gain/loss of an in vivo N-acetyl group (i.e., free amines were in vitro N-acetylated using 13C2D3-NHS acetate; introducing a 5 Da heavier acetyl moiety to each free amine [29,30]). Colored rectangles indicate the number of in vivo (black) and in vitro (orange) acetyl groups present per detected isotopic envelope.