|
Natural polymer-,
|
Polypeptide-, and Protein-based scaffolds
|
Collagen
|
groups include:
Glycine
Proline
Hydroxyproline
|
Favorable for cell adhesion, proliferation, differentiation, and ECM secretion. Excellent biocompatibility. Biodegradability. Low toxicity. Rough surface morphology. Low immunogenicity. Weak antigenicity.
|
Low mechanical strength. Difficult disinfection. The deformation and contraction of collagen-based scaffolds have restricted their use in load-bearing tissues. Poor stability in an aqueous environment. Potential for antigenicity through telopeptides.
|
[52,124,125] |
Silk fibroin
|
|
SFs are sturdy, lightweight, and have exceptional strength and elasticity. Osteoconductivity. Biocompatible. Deliver good support for cell adhesion and proliferation without initiating cell toxicity. Promote cell migration and vascularization. Moderately degradable. Thermostable (up to ∼250 °C). Commonly employed as a cell carrier for cell seeding on scaffolds.
|
|
[64,126,127] |
Fibrinogen and fibrin
|
|
Biocompatibility. High affinity for biological surfaces and molecules. Promotes cellular interactions. Variety of cell-adhesive/binding properties. Nonimmunogenicity.
|
|
[67,128,129,130] |
Gelatin
|
residues
|
Better infiltration, adhesion, spreading, and proliferation of cells on resulting scaffolds. Good stability at high temperature in a broad range of pH. Biodegradability. Osteoconductivity. Non-immunogenic. Low antigenicity.
|
|
[60,131,132] |
|
Keratin
|
|
Facilitates cell adhesion and proliferation. Unique chemistry afforded by high sulfur content. Propensity for self-assembly. Intrinsic cellular recognition. Intrinsic biological activity. Cytocompatibility. Gradual degradation.
|
|
[133,134] |
|
Polysaccharide-based scaffolds
|
Starch
|
|
Biocompatible. Thermoplastic behavior. Non-cytotoxic. Guides various developmental stages of cells. Hydrophilicity. Good substrate for cell adhesion. Good biodegradation period.
|
|
[135,136] |
Chitin/chitosan
|
|
Accelerates tissue repair. Prevents formation of scar tissue. Promotes cell adhesion. Non-toxic and non-allergenic. Bioactivity. Anti-inflammatory. Osteoconductivity. Hemostatic potential. Scaffolds could be used for a longer period. Chitosan-based scaffolds can immobilize growth factors.
|
Poor mechanical strength and stability. High viscosity and low solubility at neutral pH. Rapid in vivo degradation rate.
|
[75,137,138,139,140,141,142,143,144,145,146,147,148,149] |
Agarose
|
|
Excellent biocompatibility. Thermo-reversible gelation behavior. Exceptional electroresponsiveness. Suitable medium for cell encapsulation. Non-immunogenic.
|
|
[140,141,142] |
Alginate
|
Made up of mannuronate and gluronate monomers. Different block configurations give rise to different materials properties. Mainly made up of carboxyl groups.
|
|
|
[143,144,145] |
Cellulose
|
|
|
|
[146,147,148] |
Hyaluronic acid
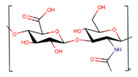
|
It is a linear, anionic, non-sulfated glycosaminoglycan with a structure composed of repeating disaccharides units: β-1,4-D-glucuronic acid and β-1,3-N-acetyl-D-glucosamide.
|
|
|
[81,149,150,151] |
Glycosaminoglycans
|
|
Biocompatibility. Anticoagulant activity. Antithrombotic activity. Anti-inflammatory. Have multiple regulatory functions, e.g., in the anticoagulation of blood, inhibition of tumor growth, and metastasis. Control the inflammatory processes.
|
|
[152,153] |
|
Synthetic polymers
|
Poly(ƹ-caprolactone) (PCL)
|
|
Controls cell proliferation and angiogenesis. Slow degradation rate (lower than that of PLA and PLGA). Non-toxic. Cytocompatibility. Good mechanical properties. Degraded by hydrolysis or bulk erosion.
|
|
[154,155,156] |
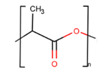
Polylactic acid (PLA)
|
|
|
|
[92,157,158] |
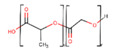
Polylactic-co-glycolic acid (PLGA)
|
|
Excellent cell adhesion and proliferation. Good mechanical properties. Features faster degradation than either PGA or PLA. Wide range of degradation rates.
|
|
[159] |
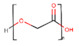
Polyglycolic acid (PGA)
|
|
|
|
[160,161] |
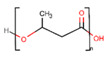
Polyhydroxybutyrate (PHB)
|
|
Non-toxic. Biostable. Biocompatible. Advantages over PLA and PGA. Slow rate of degradation. Can be obtained naturally.
|
|
[159,162,163] |
Polypropylene fumarate (PPF)
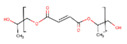
|
|
Biocompatibility. Crosslinked PPF matrices have high mechanical strength. PPF degrades in the presence of water into propylene glycol and fumaric acid, the degradation products that are easily cleared from the human body by normal metabolic processes. Non-toxic.
|
|
[159,164,165] |
Poly(ethylene glycol) (PEG)
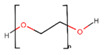
|
|
Non-ionic. Biocompatible. Elasticity. Bioadhesive. Mucoadhesive. Hinders protein adsorption. Hydrophilic. PEG as a blank template can be modified to different moieties to pass different requirements of a skin substitute like cell adhesion, short-term degradation, and minimum inflammation. Non-immunogenic.
|
its bio-inert nature.
|
[123,166,167] |
Polyurethane (PU)
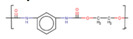
|
|
Bio- and hemocompatibility. Nontoxic. Biodegradable. Non-allergenic. Non-sensitizing. Excellent mechanical properties. High flexural endurance and fatigue resistance.
|
|
[113,168] |
Polyvinyl alcohol (PVA)
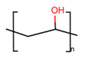
|
|
Biocompatible. Nontoxic. Noncarcinogenic. Displays a reduced protein-binding tendency, relatively higher elasticity and water content; a highly hydrated water-soluble synthetic polymer. Has relatively similar tensile strength to human articular cartilages. Good lubrication.
|
|
[169,170,171] |
Polypropylene carbonate (PPC)
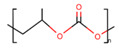
|
|
|
flow at room temperature and a relatively large brittleness at low temperature.
|
[172,173,174] |





















