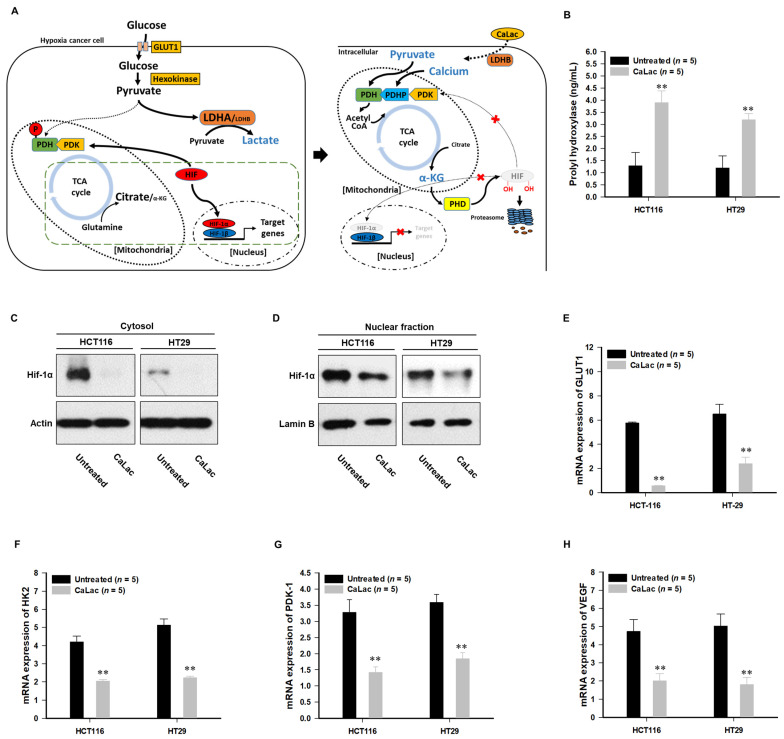
Figure 5.
Inhibition of hypoxia-inducible factor (HIF)-1α transcriptional activity by an intermediate from the tricarboxylic acid (TCA) cycle. The enzymatic reaction of prolyl hydroxylase (PHD) was increased, leading to the proteasomal degradation of HIF-1α and decreased expression of oncogenes characteristic of HIF-1α-mediated transcriptional activation. (A) Schematic illustration of HIF-1α proteasomal degradation by alpha-ketoglutarate (α-KG), a TCA cycle intermediate. α-KG is produced as a metabolite, which acts as a substrate for PHD contributing to the proteasomal degradation of HIF-1a. (B) PHD enzymatic reaction following 2.5 mM CaLac treatment in CRC cells; 2 × 106 cells were analyzed, respectively. (C,D) Proteasomal degradation of HIF-1α in the lysates of cytosol and nuclear fractions following 2.5 mM CaLac treatment in CRC cells. Uncropped blots were indicated in Figure S10. (E–H) Downregulation of glucose transporter (GLUT) 1, hexokinase (HK), pyruvate dehydrogenase kinase (PDK)-1, and vascular endothelial growth factor (VEGF) mRNA expression levels following 2.5 mM CaLac treatment in CRC cells. ** p < 0.001 vs. Untreated. Results are presented as mean ± standard deviation.