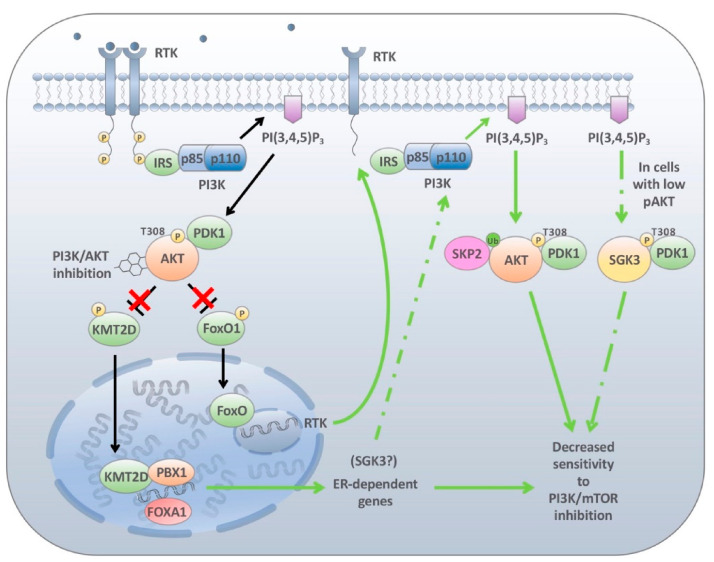
Figure 3.
Adaptive and epigenetic-driven mechanisms of resistance to PI3K/AKT inhibition. A number of adaptive mechanisms regulating PI3K inhibitor sensitivity have been described. The most notable of which is the upregulation receptor tyrosine kinases (RTKs) following PI3K/AKT inhibition. Members of the FOXO family of transcription factors are direct substrates of AKT with phosphorylation limiting their nuclear localisation. Upon AKT downregulation FOXO is drawn into the nucleus where it is recruited to binding sites which upregulate a number of receptor tyrosine kinases including HER2 and HER3. This upregulation of these RTKs results in the enhanced activity of both MAPK and PI3K signalling pathways. Similarly, in ER+ breast cancers AKT phosphorylates the methyl-transferase KMT2D inhibiting the methyl-transferase activity of the enzyme. Upon AKT inhibition KMT2D activity is restored priming the recruitment of the transcription factors FOXA1, PBX1 and ER onto ER binding sites enhancing ER-dependent gene transcription and limiting the PI3K therapeutic effects. SGK3 is an ER transcriptional target. PIK3CA mutant tumours displaying low levels of pAKT can circumvent this by activating SGK3 and downstream mTOR signalling limiting the sensitivity of PI3K inhibitors.