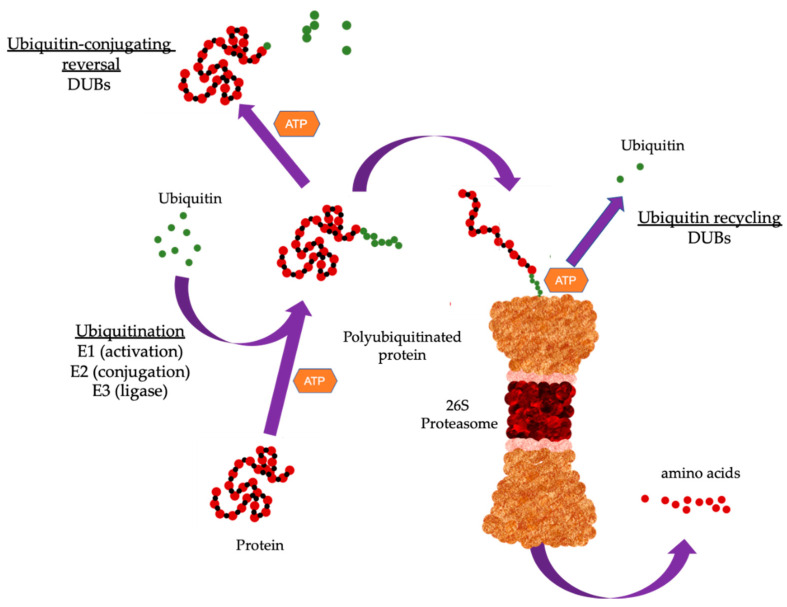
Figure 1.
Overview of the ubiquitin proteasome system (UPS). The UPS cascade. Substrate protein is ubiquitinated through the sequential action of three enzymes. E1 binds to activated ubiquitin and is transferred to the ubiquitin-conjugating enzyme (E2). The E2 carries the activated ubiquitin to ubiquitin ligase (E3), which then facilitates the transfer of ubiquitin from E2 to a lysine residue in the target protein. Proteins can be modified with a single mono-ubiquitin molecule, or with ubiquitin chains of different lengths and linkage types. Substrate proteins modified with specific chains are recognized and subsequently degraded by the 26S proteasome. Deubiquitinating enzymes (DUBs) remove ubiquitin from substrate proteins by removing mono-ubiquitination or by trimming or removing the ubiquitin chain. Typically, poly-ubiquitination has been associated with protein clearance through proteasomal degradation while mono-ubiquitination which involves the addition of a single ubiquitin moiety to the substrate protein affects cellular processes.