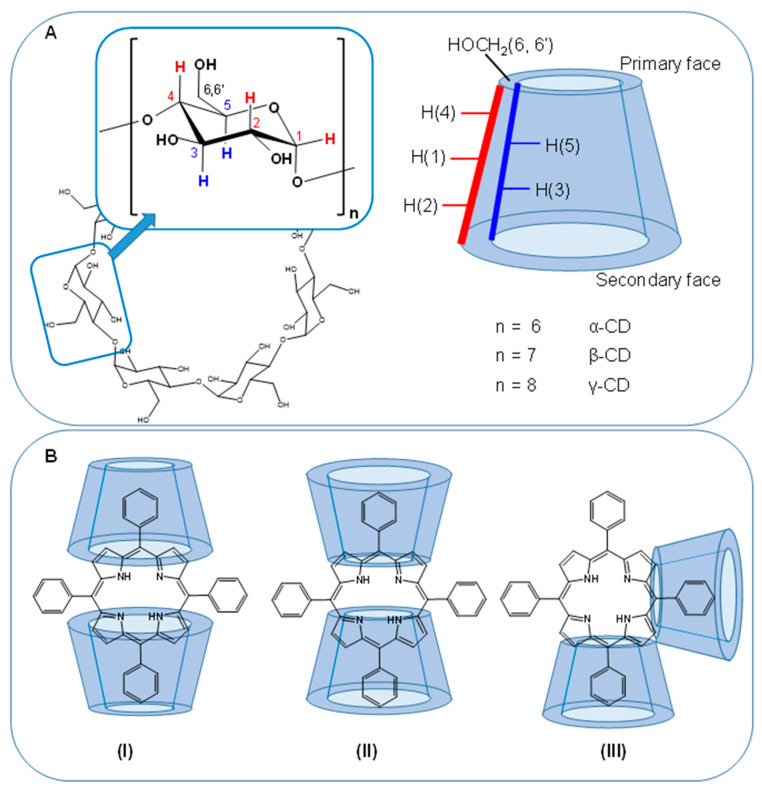
Figure 8.
(A) Structure of cyclodextrin and the α-1,4-d-glucopyranose unit with atom numbering; sketch of the cone-shaped structure of CDs in which the protons H(3) and H(5) point to the hydrophobic interior of the cavity (blue), the protons H(1), H(2) and (H4) point to the hydrophilic exterior (red), and the protons H(6,6′) are located at the rim of the primary face. (B) Examples of possible 2:1 inclusion complexes formed with tetraphenylporphyrins (TPPs): (I) inclusion via secondary face, (II) inclusion via primary face, opposite (anti) conformation, and (III) complex with adjacent (syn) conformation.