Figure 1.
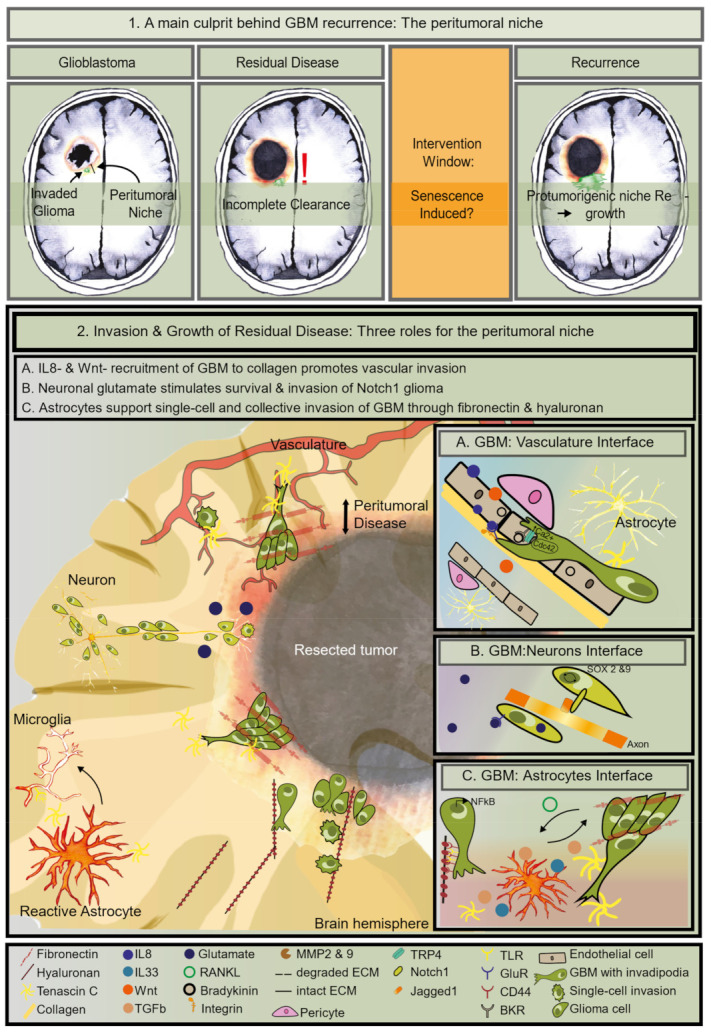
Clinical and biological definition of residual disease; (1) Illustration of different stages during patient-treatment arguing for an intervention window following standard-of-care. Treatment-induced senescence may be a target. (2) Illustration of residual disease. Graphical representation of three situations of pro-tumorigenic brain niches which can both stimulate invasion as well as promote survival of cancer cells. The left side depicts the cellular-components in the three niches and the right side shows the molecules and receptors responsible for the bi-directional communication between glioma cells and their niche. (A) IL8- & Wnt-induced invasion over blood vessels is sustained by calcium-mobilization and glioma cell cytoskeletal reorganization to move over collagen in the basement membrane. (B) Glutamate-stimulated invasion of Notch1-positive glioma cells to its cognate receptor on axons. This interaction activates SOX2 & 9 signaling in glioma cells; (C) bidirectional communication between glioma and astrocytes. NFkB is active in these glioma cells. Glioma RANKL- induced TGF-b, IL33, and MPPs secreted by reactive astrocytes are necessary for glioma invasion both as single cells and as a strand. Hyaluronan induces single cell migration through CD44 and TLR on glioma cells. Fibronectin enables collective invasion over tenascin C fibers.
