Figure 4.
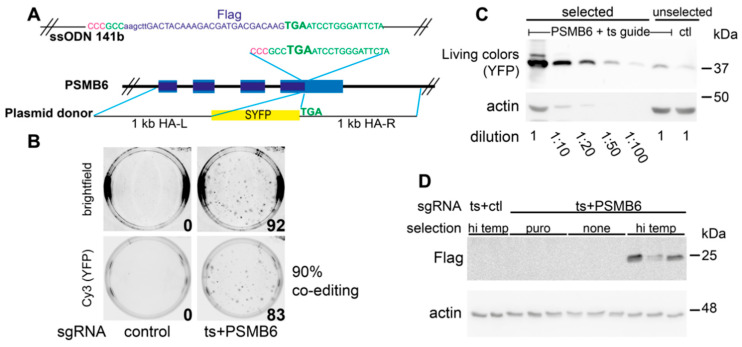
Co-editing of PSMB6 gene in HEK293: insertion of large and small tags. (A) Schematic representation of the targeted PSMB6 locus with a donor plasmid for YFP insertion and ssODN for Flag tag insertion. The stop codon of PSMB6 is indicated in bold. The guide sequence is in green, with the PAM sequence in pink (note that the guide is the minus strand). The region of the plasmid donor DNA with the 1 kb left and right homology arms (HA) and SYFP insert are shown. (B,C) Co-editing of HEK293 TAF1ts to revert ts mutation and insert YFP at PSMB6 C-terminus. HEK293 TAF1ts cells (clone 11) were transfected with donor DNA for ts correction and PSMB6-YFP, and Cas9/guide plasmids targeting TAF1ts and PSMB6 (co-edited), or nonspecific sgRNAs (control). Transfected cells were replated into 6 cm dishes and incubated at 39.5 °C. Colonies were photographed 20 days later using the Image Quant LAS 4000 system, using both the Cy3 filter to view YFP fluorescence and brightfield. (C) Cells were plated at 37 °C (unselected) or at 39.5 °C (selected), and pools of cells were analyzed by Western blotting. Dilutions of the selected cell extract were compared to the controls, showing a 50-fold enrichment of PSMB6-YFP co-editing.PSMB6-YFP is a 50 kDa protein but migrates at 37 kDa when samples are not boiled prior to loading on SDS–PAGE. This improves the detection of YFP by the Living Colors antibody. (D) Co-editing of ts and PSMB6-Flag in HEK293 TAF1ts. HEK293 TAF1 ts (clone 11) were transfected in triplicates with Cas9/guide plasmids indicated, ssODN for TAF1 and for PSMB6-Flag, and pEFIRES, a plasmid providing puromycin resistance. Two days post-transfection indicated cells were treated with 0.5 μg/mL puromycin overnight to select for transfected cells. The following day, cells were replated into 6 cm dishes and incubated at 37 °C (puro and no selection control) or at 39.5 °C. Cells were analyzed by SDS–PAGE and immunoblotting with the indicated antibodies.