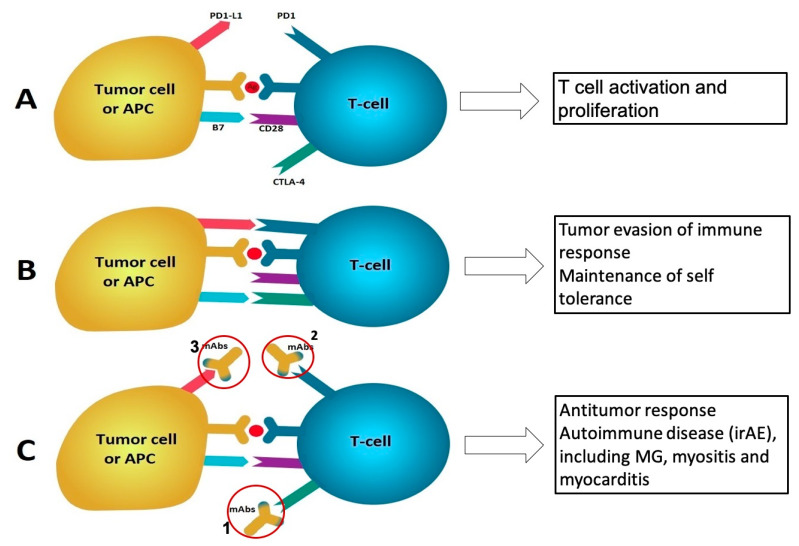
Figure 2.
Mechanism of Myasthenia gravis (MG) caused by immune checkpoint inhibitors. (A) T cell activation and proliferation start with antigen presentation to T-cells by antigen-presenting-cells (APCs); also involved in this process are major histocompatibility complex, T-cell receptors, and a costimulatory signal involving interaction between B7 (B7.1 and B7.2) on APCs and CD28 on T-cells. CTLA-4 is induced in T-cells at the time of their initial response to antigen. Activated T-cells upregulate PD-1 and inflammatory signals in the tissue induce the expression of PD1-L1. (B) B7.1 binds to CTLA-4 with greater affinity than to CD28, resulting in T-cell inactivation; the PD-1/PD-L1 signaling suppresses the activity of effector T cells in later stages of tissue inflammation. CTLA-4 and PD1/PD-L1 signaling promote self-tolerance and prevent autoimmunity. These pathways are also used by tumor cells to evade the immune response. (C) Monoclonal antibodies that block CTLA-4 or PD1/PD1-L1 pathways increase T-cell activation and proliferation which then lead to autoantibody production and increased levels of proinflammatory cytokines, causing immune-related adverse effects (irAEs) mediated by cytotoxic T cells or autoantibodies, such as myositis or MG. 1: Monoclonal antibody to CTLA-4; 2: monoclonal antibody to PD-1; 3: monoclonal antibody to PD-L1.