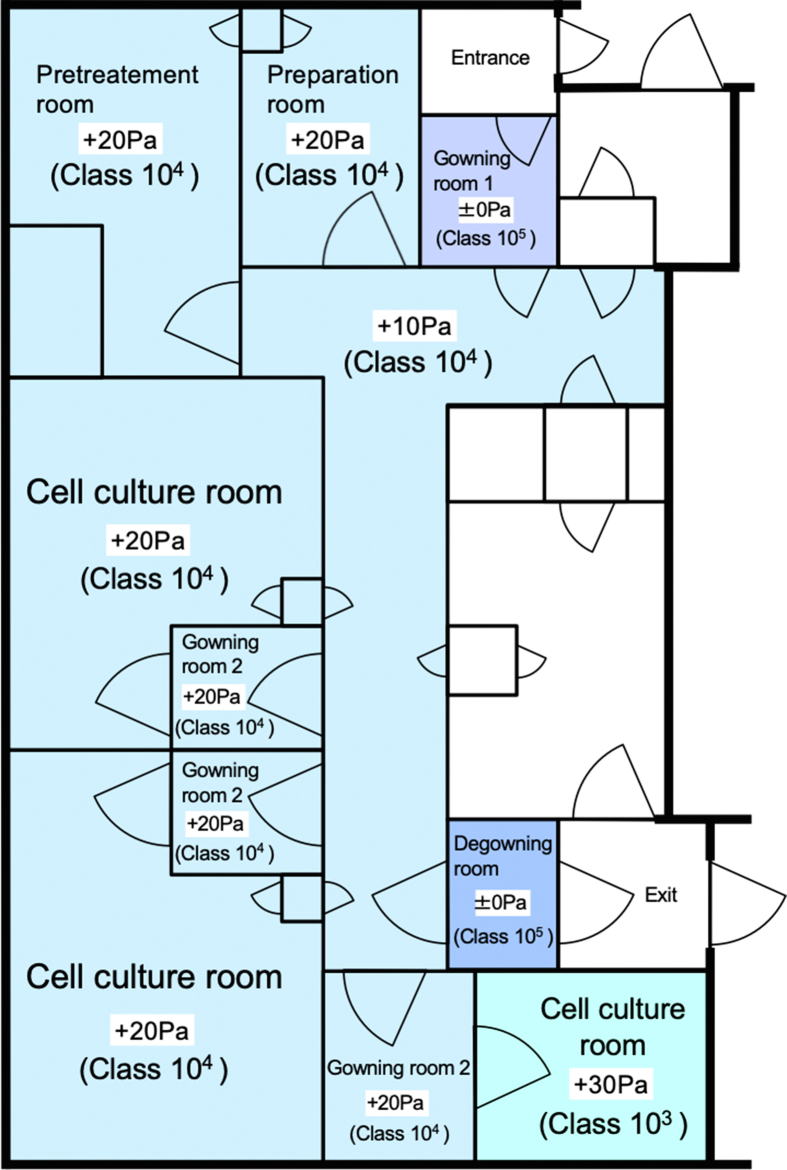
Fig. 1.
Design of the good manufacturing practice-compliant cell processing facility (CPF). In a good manufacturing practice-compliant CPF, human nasal mucosal epithelial cells were manipulated and subsequently, cell sheets were manufactured. Environment of each room in the CPF is represented in terms of atmospheric pressure (Pa) and cleanliness. Number of aerosol particles in class 103 and 104 areas were kept less than 103 and 104 particles/ft3, respectively.