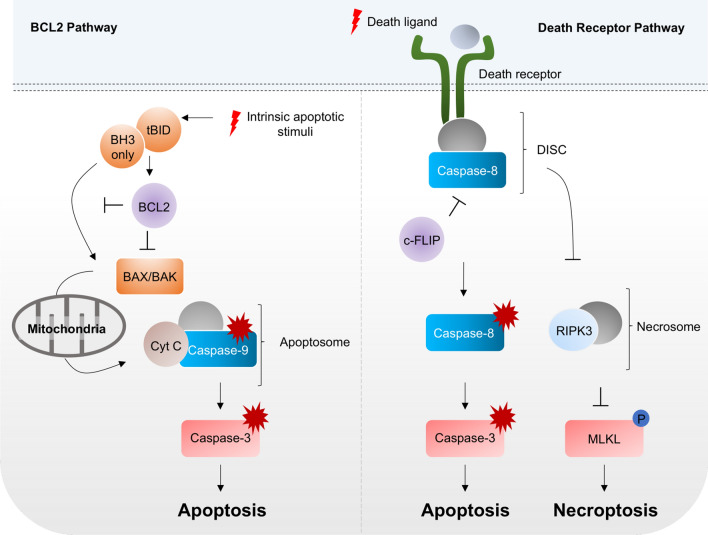
Fig. 1.
Simplified schematic overview of the BCL2-regulated and death receptor-mediated apoptosis and necroptosis signaling pathways. In the BCL2 pathway, interactions between pro- and anti-apoptotic BCL2 sub-family members determine whether the pro-apoptotic effectors BAK and BAX become activated. This results in increased mitochondrial permeability and cytochrome C (Cyt C) release, which, in turn, favors the formation of the death inducing apoptosome and ultimately Caspase-3 (CASP-3) cleavage by the initiator Caspase-9 (CASP-9). In the extrinsic pathway, binding of a death ligand to its death receptor leads to formation of the death inducing signaling complex (DISC) and activation of the initiator Caspase-8 (CASP-8). In the DISC, CASP-8 activity is restricted when CASP-8 binds to its inhibitor c-FLIP. Full CASP-8 activity in the absence of c-FLIP leads to CASP-3 cleavage, activation, and cell death by apoptosis. On the other hand, a minimal CASP-8 activity within the DISC is required to inhibit cell death via necroptosis. Loss of CASP-8 or inhibition of its enzymatic activity result in the formation of the necrosome including RIPK3, and ultimately activation and phosphorylation of the necroptosis executer MLKL. For reasons of simplicity, only key molecules of the different signaling complexes are highlighted. For further reading, we kindly refer the reader to reference [19]