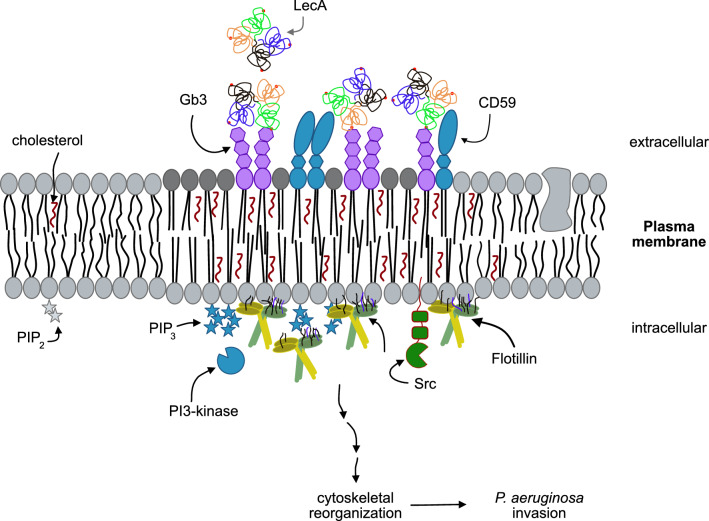
Fig. 8.
Proposed model of the interactions of LecA with the host cell plasma membrane. LecA binds to its receptor, the GSL Gb3, inducing clustering of saturated Gb3 species and recruitment of the GPI-anchored protein CD59 within the extracellular leaflet. At the intracellular leaflet PI3-kinases phosphorylate PIP2 to PIP3, which favors the recruitment of flotillins to the LecA-induced plasma membrane domain. The signal may be transduced from the extracellular to the intracellular site by transbilayer coupling between long fatty acyl chains of Gb3 and CD59 on one hand and PIPs on the other hand. Small GTPases like Rac1 and Src family kinases are known to mediate cytoskeletal reorganization. The formation of the LecA plasma membrane domain primes the host cell for an efficient uptake of PA. Components are not drawn to scale