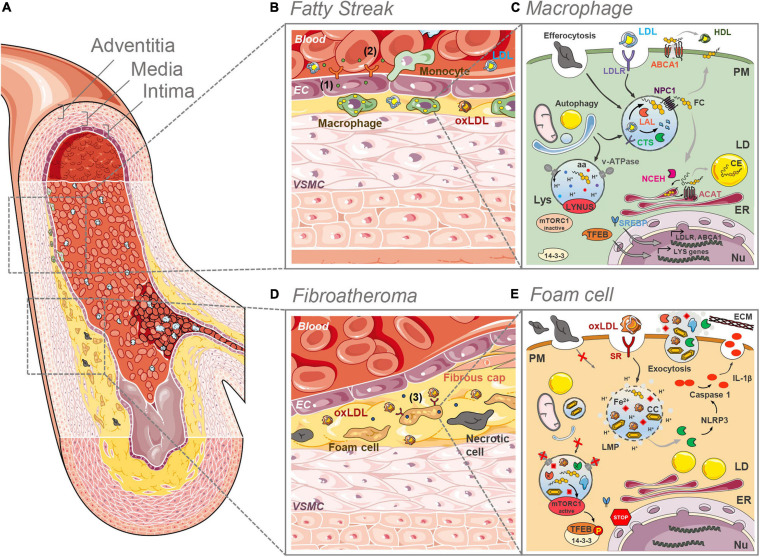
FIGURE 1.
Lysosome (dys)funtion in the initial and final stages of plaque development. (A) Anatomically, large arteries consist of three well defined and morphologically distinct layers. The intima, the innermost layer in direct contact with the blood stream, comprising an endothelial layer surrounded by a connective tissue basement membrane with elastic fibers. The media is the thickest layer and consists primarily of VSMCs. The outermost adventitia, with connective tissue and varying amounts of collagenous and elastic fibers, attaches the artery to the surrounding tissue. (B) In the initial stages of plaque development (fatty streak) LDL infiltrates the intima, where it undergoes oxidation and other modifications. The formed oxLDL causes the proliferation of VSMCs and triggers the secretion of adhesion molecules (1) and chemoattractants (2) by ECs, which in turn recruit monocytes from the blood stream. In the intima, monocytes differentiate into macrophages that clear LDL and oxLDL. (C) In macrophages of the intima the LDL is taken up by the LDLR and routed to the lysosomes (Lys) where the acid lipase LAL hydrolases the CE and TG to FC and fatty acids. The cathepsin (CTS) proteases breakdown the protein apoB. FC is exported from the lysosomes by NPC1 and then directed to the different organelles (ER, PM, etc.). The ER enzyme ACAT esterifies cholesterol to CE, which are stored in cytosolic LDs. CE can be converted back to FC by NCEH. Excess FC is effluxed via the ABCA1 plasma membrane (PM) transporter. Efferocytosis and selective autophagy converge to the lysosomal compartment for degradation of substrates sequestered in phagosomes and autophagosomes, respectively. Intralysosomal hydrolysis dependents on the activity of the proton (H+) pump v-ATPase that maintains the intraluminal acidic pH. Nutrients such as aminoacids (aa) and FC are sensed by the LYNUS machinery of which mTORC1 is a part of. Abundance of nutrients dictates the inactivity of mTORC1 and the translocation of (14-3-3) free TFEB to the nucleus (Nu) to drive the transcription of lysosome and autophagy genes. The uptake of LDL is regulated by the action of the SREBP transcription factors. (D) In advanced plaques (fibroatheroma stage) a necrotic core is formed with lipid-laden foam cells and necrotic cells. Plaque rupture is prevented by the fibrous cap formed by VSMCs that migrated from the intima. Foam cells secrete pro-inflammatory cytokines aggravating local inflammation (3). (E) As a result of the unregulated uptake of oxLDL by SRs at the surface of foam cells, the lysosomal compartment becomes overloaded with partially digested oxLDL, FC and eventually CCs. The accumulation of these materials causes lysosomal membrane permeability (LMP), the loss of the proton gradient and the release of CTS into the cytosol. There, these proteases participate in the NRLP3 inflammasome activation cascade that culminates with the processing of pro-IL-1β into IL-1β by active caspase 1. The lysosomal iron (Fe2+) pool may be one of the factors determining a decrease in the activity of the v-ATPase besides contributing to further LDL oxidation. The elevated lysosomal pH reduces the activity of the hydrolases. The decreased lysosomal hydrolytic function causes an impairment in the efferocytic and autophagic capacity of the cell. The accumulation of FC may be the culprit for the hyperactivation of mTORC1 which phosphorylates TFEB causing its retention (in a complex with 14-3-3) in the cytosol. This prevents lysosome biogenesis. Exocytosis of lysosomal contents might contribute to atherogenesis through the release to the extracellular space of partially degraded materials and the release of CTS, whose activity contributes to LDL aggregation and ECM breakdown. Some images in this figure were adapted with permission from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License (http://smart.servier.com/).