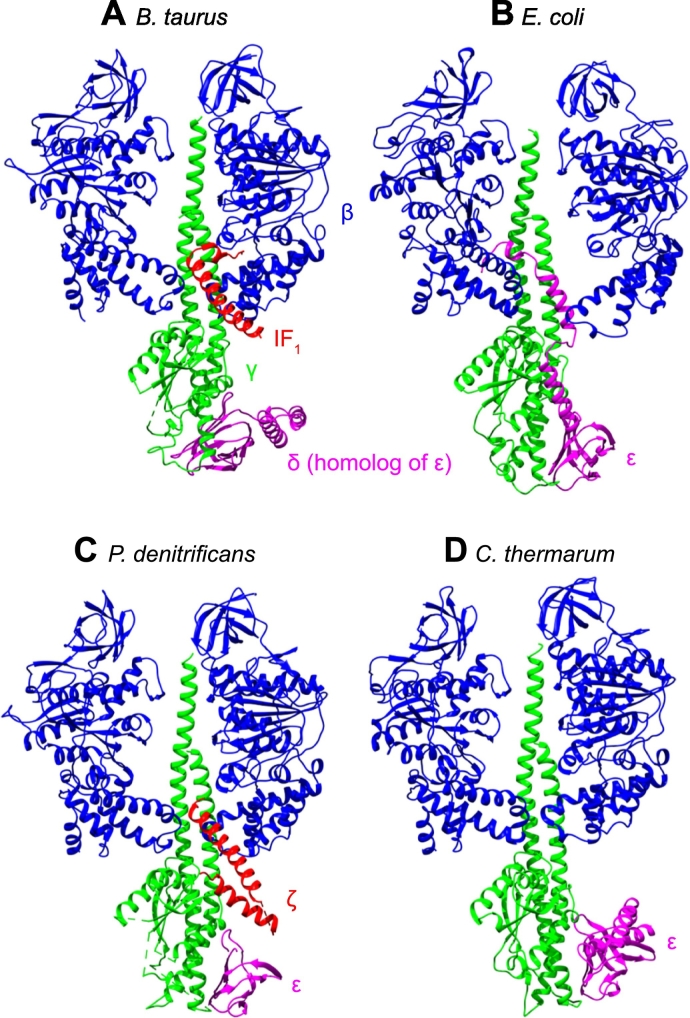
Fig. 1.
Structures of the F1 domains of four ATP synthase enzymes in states with ATP hydrolysis inhibited. For clarity, only two β subunits (blue) are shown with the γ subunit (green), bacterial ε subunit/mitochondrial δ subunit (magenta) and the unique P. denitrificans ζ subunit and mitochondrial inhibitor protein IF1 (red). (A) The F1 domain from Bos taurus with a monomeric form (residues 1–60) of the inhibitor protein IF1 bound (PDB 2V7Q) [6]. Residues 8–50 of IF1 are resolved. (B) The F1 domain from Escherichia coli with the ε subunit in the inhibitory ‘up’ state (PDB 3OAA) [11]. (C) The F1 domain from P. denitrificans with the partially resolved ζ subunit and the resolved region of the ε subunit shown; the two α-helices of the ε-CTD are not resolved (PDB 5DN6) [7]. (D) The F1 domain from Caldalkalibacillus thermarum, which is unable to hydrolyze ATP even with the ε subunit in the ‘down’ state (PDB 5HKK) [17].