Abstract
Objectives:
To test our hypothesis that pre-treatment executive function and brain regional activation during executive function would discriminate between responders and non-responders to cognitive behavioral therapy (CBT) in elderly depressed outpatients.
Design:
Clinical cohort study.
Setting:
University-affiliated hospital.
Participants:
Sixty outpatients (>age59) completed 12-weeks of CBT between July 2010 and December 2011. Forty-four completed fMRI procedures.
Measurements:
The main outcome consisted of a conversion from a clinical diagnosis (Mini-International Neuropsychiatric Interview; M.I.N.I.) of depression to no clinical diagnosis of depression or a significant improvement in diagnostic criteria. Brain activation measured by functional magnetic resonance imaging during the Wisconsin Card Sorting task was the primary predictor variable.
Results:
67% of patients had a positive response to CBT. Decreased activation in the left inferior frontal triangle and right superior frontal gyrus as well as increased activity in the right middle frontal gyrus and left superior frontal gyrus predicted a positive response to CBT. Demographic and neurocognitive measures of WCST performance were not significant predictors of a positive CBT outcome, whereas the measures of WCST-induced activity in the prefrontal cortex was a significant predictor
Conclusions:
These data are among the first to suggest that measures of prefrontal brain activation during executive functioning predicts response to CBT in older adults. Further exploration of the specific underlying processes that these prefrontal cortical regions are engaging that contributes to better CBT outcomes is warranted in larger, randomized studies.
Keywords: Depression, Cognitive-Behavioral Therapy, fMRI, Elderly
Depression in late life (LLD) contributes to increased disability and mortality in older adults (1, 2). About 8 to 16% of community-dwelling older adults report clinically significant depressive symptoms. The prevalence of major depressive disorder (MDD) is lower (1–4%) with estimates for dysthymia somewhat higher (3, 4). Many depressed elderly remain depressed regardless of treatment modality. Cognitive behavioral therapy (CBT), is a widely-used, evidence-based treatment for major, chronic depression and subsyndromal depressive symptoms (5). Its efficacy with older depressed persons is well established (6–8). While a positive response rate of about 65% has been reported (7, 8), at least one-third of older adult outpatients treated with CBT have either significant residual symptoms or remain clinically depressed (8, 9). Predicting who responds and who does not respond to CBT remains a challenge.
Cognitive function is one factor proposed to impact response to both pharmaco- and psycho-therapies (10). Between 20% and 50% of patients with LLD are estimated to have cognitive impairment greater than that in age- and education-matched controls (11), exhibiting deficits in speed-of-processing, visuospatial ability, memory, and executive functioning (11). Story et al. (12) found LLD patients with better verbal memory and faster processing speed at baseline demonstrated greater antidepressant treatment response. Also, patients with LLD who showed decrements on a semantic fluency task exhibited poorer remission rates, with executive impairment in verbal strategy explaining impaired performance (13). The authors suggest that executive functioning exerts a “top down” effect on other cognitive processes that are potentially subserved by the frontostriatal network dysfunction implicated in LLD.
The core cognitive deficits in LLD are hypothesized to reflect the significant number of cerebral deficits identified in neuroimaging investigations of this disorder (14, 15), such as increased white matter lesions, and alteration of gray matter including reduced volume of the orbitofrontal cortex, anterior cingulate gyrus, amygdala and hippocampus. Simpson et al. (16) found treatment resistant LLD patients had smaller fronto-temporal volumes and impaired immediate working memory, suggesting that those cerebral domains subserving executive function may impact treatment response. Ishizaki et al. (17) found that following pharmacotherapy, regional cerebral blood flow significantly increased in the left dorsolateral prefrontal cortex to precentral areas and the right parieto-occipital regions. While these studies underscore the value of cerebral features of LLD for predicting treatment response, all have utilized structural rather than functional MRI.
Executive function processes, including the ability to focus attention, organize, and strategize, are hallmark symptoms of LLD and well documented to be subserved by fronto-temporal brain regions (18, 19). Impairments in executive functioning may impact response to CBT given that the attention, focus, organization and strategizing required to utilize CBT effectively, are already impaired in LLD. As such, differences in the neurocircuitry subserving these functions may account for differential CBT response. We are aware of no published fMRI investigation which has examined brain regional activation during executive function as a predictor of outcome in psychotherapy for depression in older adults. Some studies have examined fMRI response to treatments for depression, including CBT, in younger patients with depression. These studies, however, have focused dominantly on apriori definition of brain regions involved in emotional regulation, with a meta-analysis implicating frontostriatal-limbic regions as having the greatest predictive potential for identifying treatment response (20). In one of the few fMRI investigations of the role of executive function, Falconer et al., (21) recently found increased activity in prefrontal networks to be associated with treatment response to CBT in younger patients with PTSD.
Given the central role of executive dysfunction in LLD, our primary aim was to examine whether patterns of prefrontal activation during the performance of an executive function task would predict response to CBT in patients with LLD.
METHODS
Forty-four subjects, aged 60 and older participated in the study. Participants were recruited through flyers describing the study. Respondents (N=156) were screened using the Patient Health Questionnaire (PHQ-9) (22). Following consent, those scoring >5 (N = 139) were administered the CES-D (23). Those with a CES-D >15 (clinical cut-off), and then meeting criteria for a primary diagnosis of major depression or dysthymia on the Mini-International Neuropsychiatric Interview (M.I.N.I.) (24, 25) were invited to participate. In some cases, participants did not meet the criteria above but had sufficient level of depression on the M.I.N.I and also self-reporting of depression on the BDI that the clinical judgment was they suffered from a sufficient level of depression that would be considered subsyndromal. Our participants thus covered the spectrum from subsyndromal through MDD. For complete socio-demographic characteristics, and depressive status and symptoms for participants see Table 1.
Table 1.
Socio-Demographic Characteristics, and Level of Depressive Symptoms for the Participants who completed the fMRI study (N=44)
| Age, Mean (SD) | 68.86(7.21) | Employment | |
| Education Level, Mean (SD) | 14.86 (2.71) | Full or Part Time (%) | 32 |
| MMSEa, Mean (SD) | 28.41 (1.48) | Not Employed (%) | 19 |
| PHQ9b, Mean(SD) | 13.70 (4.70) | Retired (%) | 41 |
| BDI-IIc, Mean(SD) | 22.52 (10.85) | Homemaker (%) | 7 |
| Depression Severity | Household Income | ||
| Subsyndromal Depression (%) | 48 | Less than $40,000 (%) | 54 |
| Major Depression (%) | 52 | $40,000 – 79,000 (%) | 21 |
| Gender | $80,000 or more (%) | 19 | |
| Female (%) | 57 | Not Reported (%) | 5 |
| Male (%) | 43 | Race | |
| Marital Status | White (%) | 92 | |
| Married (%) | 36 | Black (%) | 5 |
| Divorced (%) | 34 | Asian (%) | 3 |
| Separated (%) | 5 | Ethnic Background | |
| Widowed (%) | 14 | European Amer. (%) | 68 |
| Single (%) | 11 | Hispanic Amer. (%) | 7 |
| Current Living Situation | African Amer. (%) | 5 | |
| Living Alone (%) | 45 | Asian Amer. (%) | 13 |
| With Spouse (%) | 36 | Native Amer. (%) | 7 |
| With Children (%) | 10 | Native Language | |
| With Other Person (%) | 9 | English (%) | 73 |
| Number of Children | Other (%) | 27 | |
| 0–2 (%) | 62 | ||
| 3 or more (%) | 38 |
Mini Mental Status Exam.
Public Health Questionnaire - 9.
Beck Depression Inventory - II.
Exclusion criteria included:
1) current high risk suicidal ideation, plan and/or intent; 2) reported active psychosis; 3) reported episode of mania during the past year; 4) abusing drugs or alcohol; 5) dementia as indicated < 25 on the MMSE (26) and/or by performance on a broad battery of neuropsychological tests standardly employed for diagnosis of dementia in our center; 6) currently receiving psychotherapy; 7) preferred only medication as their treatment of choice.
A combination of the M.I.N.I. suicide risk scale, BDI-II, Beck Hopelessness Scale and the psychosocial interview from the HAM-D were used to determine if participants were actively suicidal or had high suicide risk. Those that were, were immediately referred to services, and excluded from the study. Those considered to have low to moderate levels of suicidal ideation were included, and as were all participants, were monitored continuously throughout the investigation in this regard.
Forty-four subjects, aged 60 and older, met all criteria and completed the fMRI (Table 1). At baseline, many participants reported taking medications for various physical and psychological conditions, with 83% taking at least 1 medication, 73% 2 medications, and 59% 3 or more medications. Participants were asked to try not to change their medications while in the study. Many had past psychological diagnoses, as follows: 61% past major depressive episode, 52% past MDD, 7% past minor depressive disorder, 5% minor depressive disorder recurrent, 7% past manic episode, 2% past hypomanic episode, 18% lifetime panic disorder, 5% lifetime psychotic disorder, and 7% lifetime mood disorder. Only one participant admitted to past alcoholism. In regards to current co-occurring psychological diagnoses, the following were noted: 2% current panic disorder, 14% current agoraphobia, 7% current social phobia, 7% current generalized social phobia, 2% alcohol abuse past 12 months, 2% current bulimia past 3 months, and 23% current GAD. Most of these co-occurring diagnoses are observed in depressed older adults seen in standard clinical care.
Treatment
CBT used in the current study is a 12-session individual protocol focused on skill training emphasizing behavioral activation, cognitive restructuring and social skills training. Protocol procedures were obtained from the treatment guide (6) and patient workbook (27) used in earlier studies. Therapists underwent a 6-month training program in the use of this protocol. All study therapists were supervised by experienced CBT therapists. The CBT supervisor also rated three random sessions from the beginning, middle and end of therapy, per participant by listening to recordings and rating the session on fidelity, tailored to the protocol. Supervisors gave weekly feedback to the therapists while they were treating the participants to help with adherence to the protocol (see Marquett, et al, 2013 (28)).
Measures
Primary Outcome Measures:
Diagnosis and pre-post treatment change were obtained during structured clinical interviews using the M.I.N.I. (24, 25), which has been validated against the Composite International Diagnostic Interview (CIDI) (29), and validity and reliability studies comparing it to the SCID (30) show similar results. Our definition of a positive response to treatment was complete remission or definitive evidence of an improved status in diagnosis on the M.I.N.I. following treatment. Since the M.I.N.I is more representative of standard clinical practice, and may be more objective than self-report measures of depression we believed that identification of neuroimaging predictors associated with the M.I.N.I. would be most valuable for the field. All clinicians were blind to the neuroimaging and neurocognitive assessments.
Secondary Outcome Measure:
BDI-II (31) was used to assess level of depressive symptoms pre and post intervention. It is a 21-item self-report scale that assesses somatic and psychological symptoms in the past 2 weeks. Our cut-off for a positive response to treatment was a score of 13 or less (31).
Neurocognitive Measures:
Participants were administered the Mini-Mental Status Examination (MMSE) as a screening measure of global cognitive function (26). We included the neuropsychological Wisconsin Card Sorting Test (WCST; 32) as our behavioral measure of executive functioning.
Procedures
Following determination of eligibility, participants returned for the fMRI and on a separate day the neuropsychological assessment.
fMRI measures and procedures.
fMRI scanning was performed on a GE Discovery MR750 3.0 Tesla whole body scanner (GE Medical Systems, Milwaukee, WI) using a T2* weighted gradient echo spiral pulse sequence: relaxation time = 2000 msec, echo time = 30 msec, flip angle = 80° and 1mm interleave, field of view = 220, matrix = 64×64, in-plane resolution = 3.125. Slice thickness was 4.0mm with 1.0mm gap, for a total of 30 total slices, with in-plane resolution: 3.4mm x 3.4mm. An automated high-order shimming method based on spiral acquisitions was employed to reduce field heterogeneity (33).
fMRI WCST.
Given the focus on executive functioning, the fMRI WCST was our primary predictor variable. We defined eight relevant regions of interest in the prefrontal cortex using the Automated Anatomic Labeling atlas (50). These specific ROIs are based on known anatomic boundaries, and were selected a priori to support our primary aim hypothesis because they are well documented to support executive functioning (35).
Multiple studies have successfully applied WCST-based fMRI tasks to assess prefrontal function, including the task we employ for the current study (36, 37). During the scan, participants viewed visual stimuli on a projector screen using an angled mirror and responded using a button box in the right hand. Our event-related fMRI WCST task consisted of 108 trials administered across two separate runs (6:16 mins each). During each trial, subjects were asked to sort the single card shown, by trial and error, to one of 3 target cards based on an intuited rule (color, form or number) by pressing one of the three buttons on a response box. Each trial is 3.5 seconds long with 500 msec feedback screens that indicated if the subject’s response was correct or incorrect. Variable length fixation cross (0–10sec) that followed the participant’s response resulted in a temporal jitter. The order and the length of the interstimulus intervals (4–14sec) was determined using Optseq2, an optimizing program designed to maximize the efficiency of recovery of the BOLD response (38). The sorting condition (based on color, form or number) changed after 6 stimuli irrespective of the participant’s response. Across the two functional runs, participants completed 36 trials of each of the sorting conditions. Accuracy and reaction time were also recorded.
Statistical Analyses
fMRI Data Processing.
Data from the card sorting task were preprocessed using Statistical Parametric Mapping 8 (SPM8) software (39). Images were realigned, spatially normalized and smoothed using 8mm full width half maximum. Following realignment, motion and signal intensity outliers were detected and repaired using Artifact Detection Tools software (http://www.nitrc.org/projects/artifact_detect/). Normalized, smoothed images were then submitted to random effects modeling using the general linear model as employed in SPM8 to determine effects of interest for each participant. We contrasted incorrect versus correct trials following negative feedback to examine set-shifting capacity (39). We employed a height threshold of p < 0.05 FWE corrected and extent threshold of 50 voxels. Thereafter, we defined eight regions of interest in the prefrontal cortex based using the Automated Anatomic Labeling atlas (40). Eight regions of interest were defined including bilateral inferior frontal gyrus triangle, bilateral inferior frontal gyrus operculum, bilateral middle frontal gyrus and bilateral superior frontal gyrus. These regions were selected based on their well-documented involvement in executive functions (36, 37, 41). Beta values representing the average activation in each region were extracted using the REX Toolbox (http://web.mit.edu/swg/software.htm). REX was used to extract the mean, ROI-level beta value (Table 3).
Table 3.
Multivariate logistic regression models of fMRI activity during a card sorting task predicting positive response to CBT as measured by the BDI-II and the M.I.N.I. after adjustments for demographic and Neuropsychological WSCT.
| M.I.N.I. Responders | ||||||||
| Region of Interest | B | S.E. | Wald | df | Sig. | EXP(B) | 95% CI Lower | 95% CI Upper |
| Left Inferior Frontal Operculum | 0.818 | 1.115 | 0.538 | 1 | 0.463 | 2.267 | 0.255 | 20.18 |
| Right Inferior Frontal Operculum | 0.022 | 0.792 | 0.001 | 1 | 0.978 | 1.022 | 0.216 | 4.825 |
| Left Inferior Frontal Triangle | −2.291 | 1.107 | 4.286 | 1 | 0.038* | 0.101 | 0.012 | 0.885 |
| Right Inferior Frontal Triangle | 0.53 | 0.97 | 0.298 | 1 | 0.585 | 1.699 | 0.254 | 11.372 |
| Left Middle Frontal Gy. | −2.785 | 2.144 | 1.687 | 1 | 0.194 | 0.062 | 0.001 | 4.129 |
| Right Middle Frontal Gy. | 5.777 | 2.261 | 6.531 | 1 | 0.011* | 322.801 | 3.844 | 27108.976 |
| Left Superior Frontal Gy. | 4.619 | 2.132 | 4.693 | 1 | 0.03* | 101.355 | 1.553 | 6614.83 |
| Right Superior Frontal Gy. | −7.478 | 2.977 | 6.309 | 1 | 0.012* | 0.001 | 0 | 0.193 |
| BDI-II Responders | ||||||||
| Region of Interest | B | S.E. | Wald | df | Sig. | EXP(B) | 95% CI Lower | 95% CI Upper |
| Left Inferior Frontal Operculum | −0.435 | 0.687 | 0.401 | 1 | 0.527 | 0.648 | 0.169 | 2.487 |
| Right Inferior Frontal Operculum | 0.673 | 0.68 | 0.98 | 1 | 0.322 | 1.96 | 0.517 | 7.425 |
| Left Inferior Frontal Triangle | −0.842 | 0.772 | 1.189 | 1 | 0.275 | 0.431 | 0.095 | 1.956 |
| Right Inferior Frontal Triangle | 1.178 | 1.008 | 1.366 | 1 | 0.243 | 3.247 | 0.45 | 23.399 |
| Left Middle Frontal Gy. | −2.286 | 1.555 | 2.163 | 1 | 0.141 | 0.102 | 0.005 | 2.139 |
| Right Middle Frontal Gy. | 0.635 | 1.346 | 0.223 | 1 | 0.637 | 1.888 | 0.135 | 26.408 |
| Left Superior Frontal Gy. | 2.23 | 1.382 | 2.603 | 1 | 0.107 | 9.299 | 0.619 | 139.636 |
| Right Superior Frontal Gy. | −1.06 | 1.738 | 0.372 | 1 | 0.542 | 0.346 | 0.011 | 10.456 |
| M.I.N.I. Responders Backwads Stepwise Exploratory Model Including Task Performance | ||||||||
| Variable | B | S.E. | Wald | df | Sig. | EXP(B) | 95% CI Lower | 95% CI Upper |
| Left Inferior Frontal Triangle | −1.844 | 0.667 | 7.649 | 1 | 0.006 | 0.158 | 0.043 | 0.584 |
| Right Middle Frontal Gy. | 3.48 | 1.455 | 5.722 | 1 | 0.017 | 32.452 | 1.875 | 561.644 |
| Left Middle Frontal Gy. | 1.806 | 1.005 | 3.227 | 1 | 0.072 | 6.086 | 0.848 | 43.652 |
| Right Superior Frontal Gy. | −4.047 | 1.923 | 4.428 | 1 | 0.035 | 0.017 | 0 | 0.758 |
| Constant | 1.19 | 0.601 | 3.913 | 1 | 0.048 | 3.286 | -- | -- |
Logistic regression was used for all analyses. In the primary analysis the outcome variable was based on a positive response to treatment as measured by the M.I.N.I., and for secondary analyses was based on a positive response to treatment as measured by the BDI-II. The independent variables for the primary and secondary analyses included fMRI activation in brain regions of interest (ROI) during the fMRI WCST as well as age, gender, education level and minority status.
For exploratory analysis we also included the WSCT neuropsychological measure as an independent variable, to provide insight into how important behavioral measures of executive function were as predictors of positive treatment response to CBT relative to the neuroimaging predictors. The exploratory multivariate logistic regression models were calculated using the fMRI model as a base model including brain ROIs, demographic and the WSCT neuropsychological measure. This analysis used a backward stepwise procedure to eliminate unimportant variables by eliminating variables iteratively by excluding variables with p values >.1 and retaining variables with p<0.05.
RESULTS
Primary Analyses: Within Group Whole Brain Activation:
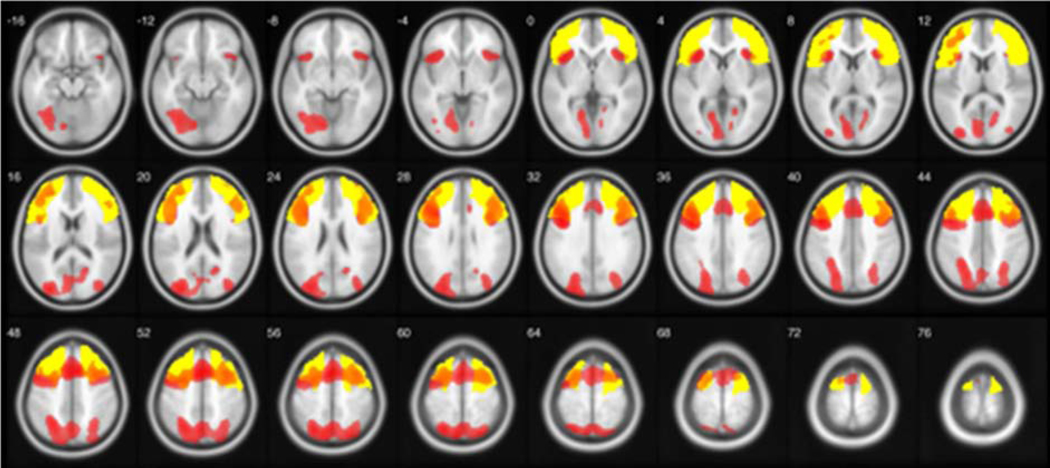
Within the total group of 44 subjects, we performed a one sample t-test analysis to validate the task as an appropriate measure of prefrontal cortex activation. We contrasted incorrect versus correct trials following negative feedback to examine set-shifting capacity. The average number of trials was 45.14 (range 26 to 66). We employed a height threshold of p < 0.05 FWE corrected and extent threshold of 50 voxels. This analysis demonstrated significant activation in left middle and superior frontal gyri, bilateral inferior frontal gyri, left medial frontal gyri, left anterior cingulate, left superior and inferior parietal lobe and left precuneus (Figure 1).
Figure 1.
Analysis of whole brain activation during a card sorting test modified to use in an MRI scanner. The incorrect versus correct trials following negative feedback activation is shown at p < 0.05, corrected. This analysis demonstrated significant activation in bilateral frontal and parietal regions. Color bar corresponds to T score.
Region of Interest-Based Logistic Regression:
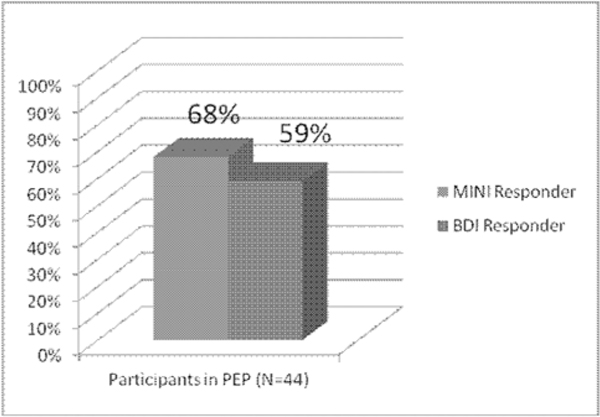
Using the M.I.N.I. to determine CBT clinical response, 30/44 (68%) of patients met criteria as a responder. Using this criteria 4 ROIs were found to be significant predictors of positive response to CBT while controlling for age, gender, education level (years) and minority status (caucasian vs. ethnic and/or racial minority). These regions consisted of the Left Frontal Inferior Triangle (LFIT), Right Middle Frontal Gyrus (RMFG), Left Superior Frontal Gyrus (LSFG) and Right Superior Frontal Gyrus (RSFG). Higher brain activity in the RMFG, and the LSFG during WCST set-shifting was associated with better CBT outcomes. Lower activity in the LFIT, and the RSFG was also associated with better CBT outcomes (Table 3).
Secondary Analysis:
Using the BDI-II with a clinical cutoff of less than 13, 26/44 (59%) of patients met criteria as a responder. Using these criteria none of the a priori ROIs were significant predictors of a positive response to CBT after controlling for age, gender, education level and minority status. However, the direction of trends was identical to those identified in the primary analysis using the M.I.N.I. (Table 3). There were no significant associations observed between our identified predictors and baseline depression severity.
Exploratory Analyses:
Using the M.I.N.I. criteria to determine clinical response and including fMRI ROIs, demographic variables and our behavioral neuropsychological measure of WCST in a single logistic regression and using a backward stepwise procedure to eliminate unimportant variables, we found that fMRI activation survived the backwards stepwise procedure whereas behavioral neuropsychological measure of WCST did not. We stress that the analysis of our behavioral neuropsychological measure of executive function is exploratory, and should be used to inform future a priori studies. In the final iteration of the backwards stepwise procedure lower activity in the LFIT and the RSFG and higher activity in the LMFG and RSFG were still significant predictors of a positive response to CBT. WCST behavioral neuropsychological measure along with age, gender, education and the other neuroimaging measures were eliminated by the backwards stepwise procedure for not contributing significantly to the overall predictive power of the model.
DISCUSSION
These results are among the first to describe fMRI predictors of positive response to CBT in patients with LLD. Baseline patterns of decreased activation in the LFIT and RSFG and increased activity in the RMFG and LSFG during an executive function task predicted better response to CBT as measured by the M.I.N.I. as the clinical metric of responders.
Several studies have implicated executive dysfunction in LLD, and several fMRI studies have demonstrated that prefrontal regions subserve executive dysfunction (19). Our study builds upon these findings, demonstrating that prefrontal activation during an executive function task predicts future response to CBT. Findings were similar when assessing response to treatment with the BDI, although the effect sizes were smaller and did not reach statistical significance.
While it may appear counter-intuitive that lower LFIT activity during WCST set shifting predicts better response to CBT, there are several potential explanations. The LFIT ROI corresponds to Broca’s area, a region with high functional lateralization to the left hemisphere that is involved in speech production. It could reflect a reduced reliance on explicit recitation of learned rules in patients that will respond well to CBT. Quicker internalization of CBT strategies so that these strategies become habitual may result in better treatment outcomes. However, it may also be that the reduced activation in LFIT and RSFG represents broad impairments in executive function neurocircuitry that are compensated for, or augmented by the executive and attentional structure of CBT, resulting in improved treatment response.
Higher RMFG activity as a predictor of better treatment, on the other, might reflect increased sustained attention and inhibitory control over previously learned rules (42). The results we present from the LSFG and RSFG shows a laterality of predictive value with higher activity in LSFG and lower RSFG predicting better CBT outcomes. SFG activity has been closely tied to the process of introspection (43). A greater brain capacity for self-monitoring activity during a set-shifting task could translate into more introspection and utilization of CBT strategies. However, increased activity in both LSFG and RSFG have both be implicated in different types of introspective tasks, leaving an open question as to what introspective processes are specific to the RSFG that could be impairing CBT treatment. Further studies are required to test these conjectures.
Our findings also suggest that fMRI activation/deactivation in frontal regions of the brain during executive functioning may be the strongest measure of response to CBT, and that behavioral measures of executive function, such as the neuropsychological measure of executive function, the WCST, may not be as sensitive. The WCST neuropsychological measure did not predict treatment response to CBT, using either outcome measure. Others have also suggested that neuroimaging measures, including fMRI, may be more sensitive measures of brain function than neuropsychological tests, particularly when considering cognitive function in older adults (44). Several studies using different neuroimaging approaches have found neuroimaging to be more sensitive to presence of cognitive impairment and to change in cognition over time than psychometric measures (45, 46). Neurocircuits subserving executive functions, captured through neuroimaging may thus be more effective at indicating who will and will not respond to CBT treatment.
This is in line with a recent investigation of behavioral predictors which also did not find the WCST neuropsychological measure of executive function predicted response to Problem Solving Therapy for LLD (47). However, these investigators did find that performance on the Stroop Color and Word Test measure of executive functioning, specifically speed-of-processing and inhibitory mechanisms, were associated with a significant reduction in depressive symptoms post-treatment. It may be that our fMRI executive function measure better captured these components of executive function than our behavioral measure of the WCST alone. Future fMRI investigations that further deconstruct the individual components of executive functioning may be required to explain these findings, and better account for own observations of differential patterns of activation predicting better CBT response.
Although there have been no fMRI studies of prediction of response to psychotherapy in older adults, Falconer et al., (21) in a younger sample of patients with PTSD, recently found deficiencies in the neurocircuits subserving executive function to predict CBT. This suggests that these neurocircuits may be relevant across a range of psychiatric disorders as well as different stages of the lifespan. Since we did not include other psychiatric disorders we are unable to speak to the specificity of our results to depression. Other limitations of our investigation include the lack of a control group or consideration of response to different treatments which may also involve executive functioning processes, such as PST. Our sample size was sufficient for examination of our primary hypotheses but was not large enough to consider subgroups of our participants based on past and current co-morbid psychiatric illness, or type of medication. Future, larger, randomized clinical trials are required to investigate these issues. Finally, given our hypothesis, we focused only on the role of executive function, restricting consideration to prefrontal brain regions implicated in this cognitive domain, and considering only the behavioral measure of the WCST. In future investigations, we will examine whether other cognitive domains or brain regions predict CBT.
In summary, our study provides the first evidence that specific patterns of fMRI activation/deactivation in frontal regions of the brain during executive functioning in older adults with depression may provide a valuable measure of response to CBT.
Figure 2.
Positive Response to Cognitive Behavioral Therapy as measured by the M.I.N.I. (improved diagnostic status following treatment) and the BDI-II (Score of 13 or less at post-treatment assessment)
Table 2:
Mean activation values for functional regions of interest (ROIs)
| Region of Interest | Mean | SD | MNI coordinate(s) of Peak Cluster(s) w/in ROI |
|---|---|---|---|
| Left Inferior Frontal Operculum (LIFO) | 1.87 | 1.7 | −44, 18, 32 |
| Right Inferior Frontal Operculum (RIFO) | 1.90 | 2.1 | 40, 14, 30 |
| Left Inferior Frontal Triangle (LIFT) | .842 | 1.9 | −42, 18, 30 |
| Right Inferior Frontal Triangle (RIFG) | .923 | 1.9 | 40, 16, 28 42, 20, 4 32, 30, 2 |
| Left Middle Frontal Gyrus (LMFG) | .335 | 1.8 | −50, 22, 34 −34, 28, 22 −38, 42, 8 |
| Right Middle Frontal Gyrus (RMFG) | 1.11 | 2.3 | 32, 12, 52 |
| Left Superior Frontal Gyrus (LSFG) | −.102 | 1.9 | −10, 22, 48 −32, 58, 24 |
| Right Superior Frontal Gyrus (RSFG) | .532 | 1.9 | 14, 14, 50 32, 60, 18 |
Acknowledgments
This study was funded by an NIMH Exploratory/Developmental Research Grant Program (R21 MH091625–01 entitled: “Predictors Of Positive Outcome In Cognitive Behavior Therapy For Late Life Depression”).
Footnotes
No Disclosures to Report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexopoulos G, Buckwalter K, Olin J, Martinez R, Wainscott C, Krishnan K. Comorbidity of late life depression: An opportunity for research on mechanisms and treatment. Biol Psychiatry 2002; 52(6):543–58 [DOI] [PubMed] [Google Scholar]
- 2.Covinsky K, Yaffe K, Lindquist K, Cherkasova E, Yelin E, Blazer D: Depressive symptoms in middle age and the development of later-life functional limitations: the long-term effect of depressive symptoms. J Am Geriatr Soc 2010; 58(3):551–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gum A, King-Kallimanis B, Kohn R: Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry 2009; 17(9):769–81 [DOI] [PubMed] [Google Scholar]
- 4.Steffens D, Fisher G, Langa K, Potter G, Plassman B: Prevalence of depression among older Americans: The aging, demographics and memory study. Int Psychogeriatr 2009; 21(5):879–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler A, Chapman J, Foramn E, Beck A: The empirical status of cognitive-behavioral therapy: A review of meta-analyses. Clin Psychol Rev 2006; 26:17–31 [DOI] [PubMed] [Google Scholar]
- 6.Gallagher-Thompson D, Thompson L: Effective treatment for late-life depression: A therapist guide. New York, NY, Oxford University Press, 2010 [Google Scholar]
- 7.Gallagher-Thompson D, Steffen A, Thompson L: Handbook of behavioral and cognitive therapies with older adults. London, Springer, 2007 [Google Scholar]
- 8.Scogin F, Welsh D, Hanson A, Stump J, Coates A: Evidence-based psychotherapies for depression in older adults. Clinical Psychology: Science and Practice 2005; 12(3):222–37 [Google Scholar]
- 9.Steffens DC, Blazer D: Mood Disorders, in 2nd edition Essentials of Geriatric Psychiatry. Edited by Blazer DG, Steffens DC. Washington, DC, American Psychiatric Assn Publishing, 2012, pp 125–148 [Google Scholar]
- 10.Ritchey M, Dolcos F, Eddington K, TJ S, Cabeza R: Neural correlates of emotional processing in depression: Changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res 2011; 45:577–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Hara R, Coman E, Butters M: Late Life Depression, in 2nd edition Clinical Neuropsychology: a pocket handbook for assessment. Edited by Snyder PJ, Nussbaum PD. 2006, pp183–231 [Google Scholar]
- 12.Story TJ, Potter GG, Attix DK, Welsh-Bohmer KA, Steffens DC: Neurocognitive correlates of response to treatment in late-life depression. Am J Geriatr Psychiatry 2008. September; 16(9):752–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morimoto SS, Gunning FM, Murphy CF, Kanellopoulos D, Kelly RE, Alexopoulos GS: Executive function and short-term remission of geriatric depression: the role of semantic strategy. Am J Geriatr Psychiatry 2011. February; 19(2):115–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mettenburg JM, Benzinger TL, Shimony JS, Snyder AZ, Sheline YI: Diminished performance on neuropsychological testing in late life depression is correlated with microstructural white matter abnormalities. Neuroimage 2012. May 1; 60(4):2182–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith GS, Kramer E, Ma Y, Kingsley P, Dhawan V, Chaly T, Eidelberg D: The functional neuroanatomy of geriatric depression. Int J Geriatr Psychiatry 2009. August; 24(8):798–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson SW, Baldwin RC, Burns A, Jackson A: Regional cerebral volume measurements in late-life depression: relationship to clinical correlates, neuropsychological impairment and response to treatment. Int J Geriatr Psychiatry 2001. May; 16(5):469–76 [DOI] [PubMed] [Google Scholar]
- 17.Ishizaki J, Yamamoto H, Takahashi T, Takeda M, Yano M, Mimura M: Changes in regional cerebral blood flow following antidepressant treatment in late-life depression. Int J Geriatr Psychiatry 2008. August; 23(8):805–11 [DOI] [PubMed] [Google Scholar]
- 18.Nyhus E, Barcelo F: The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn 2009; 71(3):437–51 [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F: Executive dysfunction and the course of geriatric depression. Biological Psychiatry 2005; (58):204–210 [DOI] [PubMed] [Google Scholar]
- 20.Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. : Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry 2004,61(9):877–889 [DOI] [PubMed] [Google Scholar]
- 21.Falconer E, Allen A, Felmingham KL, Williams LM, Bryant RA: Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder. J Clin Psychiatry 2013, 74(9):895–901 [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer R, Williams J: The PHQ-9 – Validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9):606–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radloff LS: A self-report depression scale for research in the general population. Applied Psychological Measurement 1977; (1):385–401 [Google Scholar]
- 24.Sheehan D, Lecrubier Y, Sheehan K, Amorim P, Janavs J, Weiller E, et al. : The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59:22–33 [PubMed] [Google Scholar]
- 25.Sheehan D, Lecrubier Y, Sheehan K, Janavs J, Weiller E, Keskiner A, et al. : The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry 1997; 12(5):232–41 [Google Scholar]
- 26.Folstein M, Folstein S, McHugh P: ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–98 [DOI] [PubMed] [Google Scholar]
- 27.Thompson L, Dick-Siskin L, Coon DW, Powers D: Treating late-life depression: A cognitive-behavioral therapy approach workbook. New York, NY, Oxford, 2010 [Google Scholar]
- 28.Marquett RM, Thompson LW, Reiser RP, Holland JM, O’Hara RM, Kesler SR, Stepanenko A, Bilbrey A, Rengifo J, Majoros A, Thompson DG: Psychosocial predictors of treatment response to cognitive-behavior therapy for late-life depression: an exploratory study. Aging Ment Health 2013,17(7):830–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization: Composite International Diagnostic Interview (CIDI) Version 10. Geneva, Switzerland: World Health Organization, 1490 [Google Scholar]
- 30.Spitzer RL, Williams JBW, Gibbon M, et al. : Structured clinical interview for DSM-III-R. Washington, DC, American Psychiatric Press, 1990 [Google Scholar]
- 31.Beck A, Steer R, Brown G: Manual for the Beck Depression Inventory-II. San Antonio, TX, Psychological Corporation, 1996 [Google Scholar]
- 32.Psychological Assessment Resources: Computerised Wisconsin Card Sort Task Version 4 (WCST). Psychological Assessment Resources, 2003 [Google Scholar]
- 33.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15(1):273–89 [DOI] [PubMed] [Google Scholar]
- 34.Monchi O, Petrides M, Petre V, Worsley K, Dagher A: Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci 2001; 21(19):7733–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesler SR, Kent JS, O’Hara R: Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol 2011,68(11):1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lie CH, Specht K, Marshall JC, Fink GR: Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage 2006; 30(3):1038–1049. [DOI] [PubMed] [Google Scholar]
- 37.Dale A: Optimal experimental design for event-related fMRI. Hum Brain Mapp 1999; 8(2–3):109–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashburner J: Computational anatomy with the SPM software. Magn Reson Imaging.2009; 27(8): 1163–74 [DOI] [PubMed] [Google Scholar]
- 39.Leh S, Petrides M, Strafella A: The neural circuitry of executive functions in healthy subjects and Parkinson’s disease. Neuropsychopharmacology 2010; 35(1):70–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rey A: L’examen clinique en psychologie. Paris, Presse Universitaire de France,1958 [Google Scholar]
- 41.King J: Running a best-subsets logistic regression: an alternative to stepwise methods. Journal of Educational and Psychological Measurement 2003; 63(3):392 [Google Scholar]
- 42.Nielson KA, Langenecker SA, Garavan H: Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychology and Aging 2002,17(1):56–71 [DOI] [PubMed] [Google Scholar]
- 43.Goldberg II, Harel M, Malach R: When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron 2006, 50(2):329–339 [DOI] [PubMed] [Google Scholar]
- 44.Zamrini E, De Santi S, Tolar M: Imaging is superior to cognitive testing for early diagnosis of Alzheimer’s disease. Neurobiology of Aging 2004, 25:685–691 [DOI] [PubMed] [Google Scholar]
- 45.Arnaiz E, Jelic V, Almkvist O, Wahlund LO, Winblad B, Valind S, et al. : Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport 2001,12:851–855 [DOI] [PubMed] [Google Scholar]
- 46.Cardenas VA, Du AT, Hardin D, Ezekiel F, Weber P, Jagust WJ, et al. : Comparison of methods for measuring longitudinal brain change in cognitive impairment and dementia. Neurobiol Aging 2003, 24:537–544 [DOI] [PubMed] [Google Scholar]
- 47.Mackin RS, Nelson JC, Delucchi K, Raue P, Byers A, Barnes D, Satre DD, Yaffe K, Alexopoulos GS, Arean PA: Cognitive Outcomes after Psychotherapeutic Interventions for Major Depression in Older Adults with Executive Dysfunction. Am J Geriatr Psychiatry 2013,(13):413. [DOI] [PMC free article] [PubMed] [Google Scholar]